ATCC introduces new product segment with launch of SARS-CoV-2 external control kit
December 4, 2020
Source: drugdu
 517
517
ATCC, the world’s premier biological materials management, and standards organization, today announced the introduction of its new SARS-CoV-2 External Control Kit for clinical laboratories and test manufacturers.
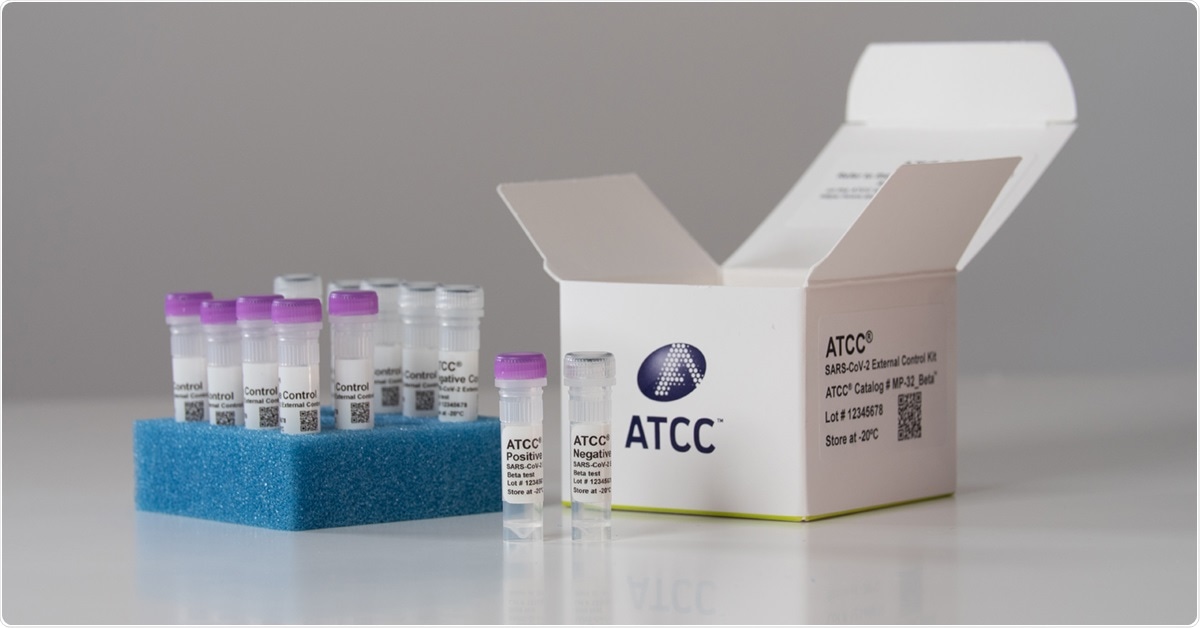
Image Credit: ATCC
The ready-to-run kit provides controls in the rapidly changing COVID-19 testing landscape as a workflow-optimized kit with complete genome coverage that’s widely compatible with most commercial and laboratory-developed tests.
Federal regulation requires that clinical laboratories assess the performance of their testing applications. This new ATCC kit is a practical, kitted solution clinical laboratories can use to meet this requirement. Validated, convenient, and ready-to-use, it offers a universal, full-process positive control (a heat-inactivated virus) and negative control (human cell line) that allow clinical laboratories to easily and accurately verify and assess the quality of their molecular diagnostic assays.
The SARS-CoV-2 External Control Kit is compatible with most molecular diagnostic tests being used today. This broad compatibility is critical as surveys from the Association for Molecular Pathology have shown that supply shortages and other factors have led most clinical laboratories to perform COVID-19 diagnostic testing using more than one commercially available and/or laboratory-developed test.
The kit includes positive and negative controls that can be used to determine the performance quality of the lab’s entire testing workflow, including sample extraction and target amplification, regardless of which molecular diagnostic test is being used.
“The positive and negative controls are, as usual, high-quality products from ATCC. These provide reliable and safe process controls for use across the wide range of settings where SARS-CoV-2 molecular testing will be performed,” said Jesse J. Waggoner, M.D., Assistant Professor, Department of Medicine, at Emory University School of Medicine.
About ATCC
ATCC is a premier global biological materials and information resource and standards organization and the leading developer and supplier of authenticated cell lines and microorganisms. With a history of scientific advancements spanning nearly a century, ATCC offers an unmatched combination of being the world’s largest and most diverse collection of biological research solutions and a mission-driven, trusted partner that supports and encourages scientific collaboration.
ATCC products, services, and people provide the scientific community with credible biological products, advanced model systems, and custom solutions that support complex research in a variety of innovative applications resulting in incredible achievements in basic science, drug discovery, translational medicine, and public health.
ATCC is a non-profit organization with headquarters in Manassas, VA, and an R&D innovation center in Gaithersburg, MD.
From:https://www.news-medical.net/news/20201204/ATCC-introduces-new-product-segment-with-launch-of-SARS-CoV-2-external-control-kit.aspx
By editorRead more on
- Bio-Thera Solutions’ innovative drug, Darpubayimab injection, has had its marketing authorization application accepted by the NMPA February 25, 2026
- Dashi Pharmaceuticals licenses its migraine medication to NewCo in the United States. February 25, 2026
- United Laboratories subsidiary, UBT251 injection (a Class 1 innovative drug), achieved near-20% weight loss in Phase II clinical trials February 25, 2026
- Dongyangguang Pharmaceutical’s first anti-Nipah virus “lifesaving drug” has entered clinical trials, achieving a 100% mortality protection rate in animal prevention. February 25, 2026
- 34 million patients no longer rely on imported drugs February 25, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



