Yiming Biotech Completes RMB 200 Million Pre-D Round of Financing
January 1, 2025
Source: drugdu
 381
381
Recently, Jiangsu Yiming Biotechnology Co., Ltd. (hereinafter referred to as "Yiming Biotech") announced the successful completion of a new round of strategic financing of nearly RMB 200 million. This Pre-D round of financing was jointly invested by Beijing Changping Industrial Development Investment Fund and Beijing Pharmaceutical and Health Industry Investment Fund. It is another round of financing obtained by Yiming Biotech after the C+ round of financing in April 2023.
Yiming Biotech said that as a bridge for ATMPs to go global and commercialize, it has been recognized and strongly supported by multiple investors in the current complex market environment. This round of financing will be used to further support the construction of Yiming Biotech's ATMPs CDMO commercial production base, promote technology iteration, enhance the service capabilities of the CDMO global service network, consolidate the company's core competitiveness in the ATMPs drug technology track, and create a large CDMO service platform from preclinical research, product development to commercial production.
Founded in 2015, Yiming Biotech is a research and development and production biotechnology company dedicated to the development and application of ATMPs technology and providing one-stop CDMO overall solutions for innovative drug companies. Yiming Biopharma is based in China and has a global presence. It has established nearly 20,000 square meters of GMP production bases in Maryland, the United States, Suzhou, Jinan, Guangzhou, Beijing, China, and other places. It has nearly 50 GMP production lines worldwide, and has established CMC global R&D centers in Vancouver and Nanjing, providing one-stop CDMO services such as GMP plasmids, viral vectors, cell therapy drugs, and RNA drugs.
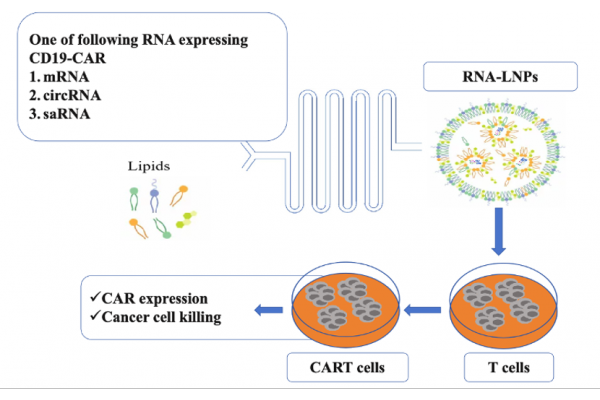
At the 66th Annual Meeting of the American Society of Hematology (ASH 2024) held earlier this month, Yiming Biopharma introduced a new RNA-LNP technology in the form of a poster, which can effectively and safely deliver therapeutic RNAs such as messenger RNA (mRNA), circular RNA (circRNA), and self-amplifying RNA (saRNA) into T cells, which not only simplifies the production process and improves the safety of treatment, but also provides a multifunctional platform for the development of next-generation cell immunotherapy.
At present, Yiming Biopharma has a professional registration and application team in both China and the United States, which can help customers complete dual-application projects in China and the United States in one stop. There are currently dozens of successful registration and application cases, and many projects have entered Phase III clinical trials. Yiming Biopharma's sound global R&D and production service network, leading technological advantages, and experienced professional team are dedicated to providing global ATMPs companies with full-process process development and production preparation services from early R&D to commercial production.
Mr. Li Qichen, founder and CEO of Yiming Biopharma, said: "We sincerely thank Beijing Changping Industrial Development Investment Fund and Beijing Pharmaceutical and Health Industry Investment Fund for their trust and recognition. This financing will further enhance Yiming Biopharma's independent innovation capabilities and core competitiveness, and is a solid step taken by Yiming Biopharma on the road to promoting the innovative development of the pharmaceutical and health industry. Since its inception, Yiming Biopharma has been constantly making progress and accumulating strength. With continuous technological innovation, high-quality products and services, and stable business growth, it has won wide recognition from the capital market. With the successful completion of this round of Pre-D financing, Yiming Biopharma will embark on a new journey, accelerate the construction of a commercial production base in Beijing, and continue to promote global strategy and technological innovation. In an era when the ATMPs industry is growing and commercialization is accelerating, we will focus on technological advantages and better support global ATMPs companies with a complete global CDMO service network and infrastructure."
https://news.yaozh.com/
By editorRead more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



