Two innovative medical devices were approved for marketing
November 14, 2024
Source: drugdu
 257
257
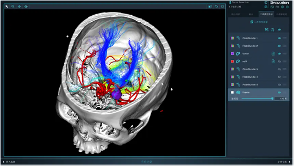 Recently, the National Medical Products Administration announced the approval of two innovative product registration applications: "Brain Surgery Planning Software" by Beijing Huake Precision Medical Technology Co., Ltd. (hereinafter referred to as Huake Precision) and "Implantable Deep Brain Neurostimulation Extension Lead" by Boston Scientific Neuromodulation Company (hereinafter referred to as Boston Scientific).
Recently, the National Medical Products Administration announced the approval of two innovative product registration applications: "Brain Surgery Planning Software" by Beijing Huake Precision Medical Technology Co., Ltd. (hereinafter referred to as Huake Precision) and "Implantable Deep Brain Neurostimulation Extension Lead" by Boston Scientific Neuromodulation Company (hereinafter referred to as Boston Scientific).
Huake Precision focuses on the research and development, production and technical services of intelligent and innovative medical devices. It is a leading company in China's innovative neurosurgery medical devices, providing comprehensive technology and product solutions for the treatment of brain diseases.
It is reported that Huake Precision currently has five major product lines including the SR series of neurosurgery robots, the Q300 series of micro neurosurgery robots, the NS series of neurosurgery navigation, the Huake Hengsheng intracranial electrode series, and the LS series of magnetic resonance guided laser ablation treatment systems. It has built a full-chain technology system including underlying algorithms, image processing, artificial intelligence, optical ablation, navigation positioning, and ultra-precise signals, laying a solid foundation for the c头omprehensive and comprehensive development of China's high-end medical devices, and forming an integrated platform for equipment and consumables covering the entire surgical process such as intelligent diagnosis and treatment with multi-modal robots working together. At the same time, it also brings internationally leading comprehensive technology and product solutions to the treatment of brain diseases in China's neurosurgery.
The brain surgery planning software SinoPlan™ approved this time consists of a software installation program and an authorization file. The functional modules include: user login, patient sequence management, surgical planning, image registration, three-dimensional reconstruction, head frame parameter calculation, and fiber bundle generation, which are used to formulate brain surgery plans.
This product uses multi-dimensional space vascular reconstruction and avoidance technology. By combining existing headrest tools and planning paths, it can optimize brain surgical strategies for cerebral hemorrhage, brain tumors, cerebrovascular diseases, epilepsy, Parkinson's disease, etc., and improve clinical outcomes. Work efficiency and surgical efficacy.
Huake's precise brain surgery planning software SinoPlan™ has reached the international leading level in key technologies such as multi-modal image data processing, segmentation and fusion, registration and registration, and brain drift correction. It solves the problem of existing domestic surgical planning software and leksell head frame, Navigation and surgical robots are not compatible at the same time. It provides neurosurgeons with a powerful, more efficient, more intelligent and reliable assistant, especially for minimally invasive surgeries on small intracranial lesions, deep lesions, multiple lesions and lesions located in important functional areas. Positioning and precise resection have significant clinical value.
As an important tool for doctors to assist diagnosis and decision-making, neurosurgery planning software has broad market application prospects. After this product is launched, it will not only achieve import substitution and reduce the cost of using surgical planning software, but will also significantly shorten doctors’ experience in frame and frameless three-dimensional The learning curve of directional surgery improves the level of precise diagnosis and treatment of neurosurgery in China.
It is worth mentioning that the brain surgery planning software was approved to enter the national innovative medical device special review process in March 2022. It is the first brain surgery planning software with independent intellectual property rights in the country, and it is also the fifth model of Huake Precision approved for listing. National innovative medical device, the 11th Class III medical device registration certificate. Moreover, this product is also the 300th approved innovative medical device.
Boston Scientific's implantable deep brain neurostimulation extension lead is Boston Scientific's first product in the world. Combined with the exclusive MICC (Multiple Independent Current Control) technology, it can achieve precise control of the direction, position, range and amplitude of electrical stimulation, and conduct personalized parameter settings for different patients and different stages of disease progression of the same patient, so as to accurately stimulate the target point and avoid the side effect area, bringing better treatment effects to patients and relieving their symptoms more continuously and stably.
The implantable deep brain neurostimulation extension lead consists of electrodes and cranial hole electrode lock covers and supplementary tool kits, tunnel tools, torque wrenches and connecting cables. By conducting electrical stimulation pulses, it can stimulate the bilateral subthalamic nucleus and the internal part of the globus pallidus, and is used for auxiliary treatment of symptoms of mid-to-late levodopa-responsive Parkinson's disease.
In addition, the product uses a combination of terminal electrodes, directional electrodes and multiple independent currents, which can effectively increase the treatment window of deep brain electrical stimulation, improve the accuracy of target positioning and the cure rate, and reduce the risk of re-surgery to implant electrodes. The directional electrode lead has 8 electrode contacts, which segment the second and third electrodes at the distal end of the traditional 4-contact electrode, allowing it to be directionally adjusted.
It is worth mentioning that the 7th CIIE has just ended, and Boston Scientific also brought a highlight of the neurological field at the conference - the Vercise Genus implantable deep brain neurostimulation (DBS) system.
The system introduces MICC (Multiple Independent Current Control) technology, achieving sub-millimeter stimulation accuracy, and has a unique directional electrode design. Combined with the advantages of MICC technology, it can flexibly "shape" the range of nerve stimulation, assisting doctors in stimulating the target points more accurately in the complex structure of the brain, avoiding areas that produce side effects, and alleviating symptoms such as tremors, slow movements, and stiffness. While improving the motor function of Parkinson's disease, it can adjust the treatment method according to the development of the patient's Parkinson's disease, and has an ultra-long service life of 25 years.
As a new generation of DBS products, Vercise Genus is rechargeable, MRI-compatible, one of the smallest pulse generators on the market, and has a Bluetooth link function, which can achieve wireless thread control.
https://news.yaozh.com/archive/44526.html
By editorRead more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



