Tsinghua University, in collaboration with Minhai Biotechnology, discovers a novel immunoprotective mechanism for vaccine clearance of pathogenic bacteria
January 4, 2024
Source: drugdu
 364
364
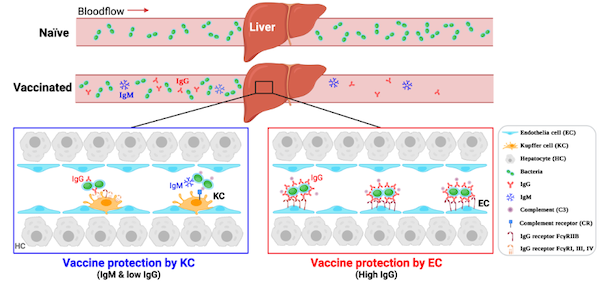
Vaccine immunization has dramatically reduced the morbidity and mortality of invasive bacterial infectious diseases such as bloodstream infections. Although a large number of studies have demonstrated that immune protection requires vaccine-induced antibody production, human beings still lack a clear understanding of the cellular and molecular mechanisms of how vaccines provide immune protection. The current international consensus is that vaccines provide immune protection by activating phagocytes in the spleen and blood circulation to remove pathogens that invade the bloodstream.
Recently, Jingren Zhang's group and Linqi Zhang's group at Tsinghua University School of Medicine, together with Haifa Zheng's group at Beijing Minhai Biotechnology Co., Ltd, published a paper in Science Translational Medicine titled "Liver macrophages and sinusoidal endothelial cells The research paper, entitled Liver macrophages and sinusoidal endothelial cells execute vaccine-elicited capture of invasive bacteria, reveals that the liver is the main organ that executes vaccine-elicited immunoprotection, and elucidates the molecular mechanism by which vaccine-elicited liver macrophages and hepatic sinusoidal endothelial cells are activated by vaccine to resist invasive bacterial infection.
In this study, the researchers used the 23-valent pneumococcal polysaccharide vaccine (PPV23) and 13-valent pneumococcal polysaccharide conjugate vaccine (PCV13) produced by Beijing Minhai Biotechnology Co., Ltd. to establish a mouse model of post-vaccine immunization attack, and traced that the vaccinated individuals could capture in the liver the pathogenic bacteria that could not be cleared originally in the blood circulation in a very short period of time. Further at the cellular level, it was confirmed, including by in vivo real-time imaging of mouse livers, that vaccine-induced antibodies enable hepatic resident macrophage blight cells to capture and kill pathogenic bacteria that would otherwise be unrecognizable and uncaptured by the host. The blight cells were able to capture and kill both IgG-coated bacteria via the antibody receptor FcγR and pathogens whose IgM antibodies were conditioned to bind to complement C3 proteins via the complement receptors CRIg and CR3. Unexpectedly, the potent PCV13 vaccine capable of inducing high levels of IgG antibodies could further enable hepatic sinusoidal endothelial cells to bind pathogenic bacteria in the circulation via the low-affinity antibody receptor FcγRIIB, which mechanistically elucidated the vaccine to have a better protective effect. The above mechanism of vaccine protection based on liver immune function is also applicable in the vaccines against two other important invasive human pathogens, Neisseria meningitidis and Klebsiella pneumoniae.
In summary, the present work provides the first in-depth and systematic explanation of the mechanism of vaccine immunoprotection in the international arena, and also provides a new theoretical basis for future vaccine development and evaluation.
https://mp.weixin.qq.com/s/MiaROOlEZnbbxpCJKx0Pcg
By editorRead more on
- Marketing application for HSK39297 tablets, an innovative drug for paroxysmal nocturnal hemoglobinuria, has been accepted March 9, 2026
- Clinical trial application for olopatadine-mometasone nasal spray approved by NMPA March 9, 2026
- Superior to standard therapy! Johnson & Johnson’s blockbuster new drug combination therapy receives FDA approval March 9, 2026
- For the first time, a domestically produced ultra-long-acting TSLP bispecific antibody has been approved for clinical trials. March 9, 2026
- With Hengrui entering the fray, can Mingrui hold its ground? March 9, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



