The king of cancer is to be defeated
November 12, 2024
Source: drugdu
 585
585
 Pancreatic cancer is known as the "king of cancer" because of its high malignancy and lack of effective drug treatment. However, the research and development community has never stopped its efforts to conquer this type of cancer.
Pancreatic cancer is known as the "king of cancer" because of its high malignancy and lack of effective drug treatment. However, the research and development community has never stopped its efforts to conquer this type of cancer.
Recently, Hengrui Medicine announced that the results of the Phase III study of irinotecan liposome (II) combined with advanced pancreatic cancer were published in the Nature sub-journal "Signal Transduction and Targeted Therapy". The results showed that the irinotecan liposome combination regimen reduced the risk of death in pancreatic cancer patients by 37% compared with the control group.
In the next era beyond chemotherapy, perhaps ADC drugs will become the main force in the fight against pancreatic cancer. For example, Innovent Biologics' ADC targeting cluadin18.2 and Lepu Bio's ADC targeting EGFR each have their own surprises, and both have shown breakthrough efficacy in the remission rate of pancreatic cancer.
In addition, Kangfang has also made breakthroughs in immunotherapy for pancreatic cancer and explored the treatment of pancreatic cancer with dual antibodies.
The gap in the king of cancer is getting bigger and bigger.
Pancreatic cancer and innovative chemotherapy
The prognosis of pancreatic cancer, the "king of cancer", is extremely poor, with a very short survival period and a 5-year survival rate of about 10%. This type of cancer has no obvious symptoms in the early stage, and is discovered in the late stage, and the treatment methods for the late stage are also very limited.
According to the diagnosis and treatment guidelines issued by the Chinese Society of Clinical Oncology (CSCO), for advanced pancreatic cancer, chemotherapy is the main means of treatment, whether first-line or second-line. For the first line, the main treatment is gemcitabine combined with albumin paclitaxel, and the second line is nanoliposomal irinotecan.
At present, immune checkpoint inhibitors such as PD-1 have not been approved for this type of cancer, and the advancement of various types of macromolecular therapies has encountered very large obstacles, which is closely related to some characteristics of pancreatic cancer itself.
According to the review "Progress in the Study of PD-1/PD-L1 Immune Checkpoint Inhibitors for the Treatment of Pancreatic Cancer", most types of pancreatic cancer are considered to be static immune or resistant tumors, because the clinical effect and sensitivity of PD-1/PD-L1 immune checkpoint inhibitors in the treatment of pancreatic cancer are highly dependent on the tumor immune microenvironment and the expression level of PD-L1.
However, the cells that play an immune role are mainly distributed in the peritumoral stroma, and very few are distributed in the tumor substance. The interstitial tissue of pancreatic malignant tumors is poorly vascularized, and the tumor stroma is rich in fibroblasts and contains a large amount of desmoplastic matrix.
In this case, not to mention that immune-related cells such as T cells find it difficult to enter the tumor substance from the stroma, even large molecule drugs find it difficult to enter, which makes it difficult for PD-1 monoclonal antibodies to exert their satisfactory effects.
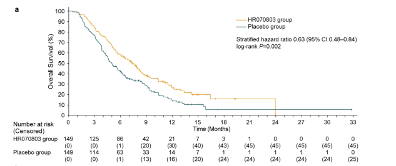
Innovation in the dosage form of chemotherapy small molecule drugs and strengthening the penetration of drugs into the tumor were important directions for the development of pancreatic cancer therapy in recent years. Irinotecan liposome injection ONIVYDE was first developed by Academic Medical Center and HERMES BioSciences. Later, Pharma Engine in Taiwan, China introduced the Asian regional rights. Recently, Hengrui has improved on the basis of the original research formula and developed an innovative irinotecan liposome-HR070803.
This liposome has made further breakthroughs based on the predecessors, making its drug release more persistent and less likely to be absorbed by the reticuloendothelial system. By increasing the time in blood circulation, it increases the possibility of penetration into tumor tissue. Compared with the original drug, the differentiated advantage of this drug is that the molecular particle size is smaller (the diameter of ONIVYDE molecule exceeds 100nm, while the diameter of this molecule is 80-90nm), which is more conducive to penetration into the tumor. According to
Hengrui's Phase III clinical data of this drug, the OS of the treatment group was 7.4 months, while the OS of the placebo group was 5 months, and the HR value reached 0.63, and the patients in the treatment group benefited significantly.
The benefit of progression-free survival was even more obvious: the mPFS of the treatment group was 4.2 months, and the mPFS of the placebo group was 1.5 months, with an HR of 0.36.
ADC are the continuation of chemotherapy. Relying on the bystander effect, perhaps ADC drugs can have an unexpected therapeutic effect on this type of cancer.
Innovent's claudin18.2-targeted ADC-IBI343 has announced preliminary efficacy, which is surprising.
The current structure of Innovent's IBI343 is relatively clear. Its antibody is HB37A6. The antibody affinity is one order of magnitude higher than Zolbetuximab (IMAB362, claudin18.2 monoclonal antibody). The linker uses synaffix sugar-specific site-specific coupling technology. The core differentiation of its platform lies in the hydrophilicity of the linker, which is the main direction of ADC development at present. Increasing hydrophilicity helps to prolong the blood circulation time of ADC drugs and helps to prolong the duration of drug effectiveness. Similar to the liposome dosage form innovation mentioned above, it is to increase the possibility of ADC drugs reaching tumors.
In terms of payload, IBI343 uses exatecan. Like DXD, both are derivatives of camptothecin toxins. The structural difference mainly lies in the difference between the amino group and the amide + hydroxyl structure on the alkane ring. Of course, the main active structure of camptothecin toxins lies in the chiral structure of the lactone ring and hydroxyl at the bottom. The difference in structure makes the two different in toxicity. Exatecan is much more toxic than DXD, which makes the DAR value design of the two different. The DAR value of Innovent's IBI343 is 4, while the DAR value of Daiichi Sankyo's ADC drug with DXD as payload, such as DS-8201, is 8. This is to improve the safety of IBI343 and expand the treatment window.
Due to the site-specific coupling of sugars, IBI343 can be said to be strong in its stability, but in terms of the killing of bystander effects, it may not be as good as Dxd. Each has its own advantages and disadvantages.
Innovent's IBI343 can be said to be born for gastrointestinal tumors. The target of claudin18.2 is designed to deal with various types of gastrointestinal cancers, but the related monoclonal antibodies of the previous era did not break through the shackles of pancreatic cancer. But now IBI343 shows hope.
At ASCO this year, IBI343 showed preliminary efficacy in the treatment of advanced pancreatic cancer.
At the patient baseline, 35 patients with advanced pancreatic ductal adenocarcinoma (PDAC) or biliary tract cancer (BTC) were enrolled. All subjects had previously received at least 1 line of treatment, with a median number of treatment lines of 2. In other words, the median number of lines of treatment for patients has been treated with irinotecan liposomes.
In terms of treatment effect, among the 25 subjects who had undergone at least 1 post-baseline tumor assessment, 7 achieved partial remission (PR), including 5 PDAC patients. In the 6 mg/kg dose group, among the subjects with CLDN18.2 IHC1/2/3+≥60%, 13 had at least one post-baseline tumor assessment, of which 5 subjects achieved PR and ORR reached 38.5%.
In terms of safety, 25.7% of the subjects experienced grade 3 or higher treatment-related adverse reactions, which is acceptable for ADC.
Currently, it has been granted fast track status by the FDA.
https://news.yaozh.com/archive/44518.html
By editorRead more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



