Silicon Chip Based Blood Test Rapidly Diagnoses Patients with Hypothyroidism
July 31, 2024
Source: drugdu
 388
388
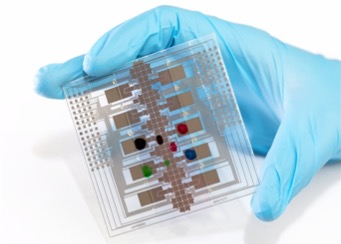 Hypothyroidism impacts about 10% of the U.S. population, making the Thyroid Stimulating Hormone (TSH) test the most frequently conducted immunoassay in the United States. Traditional testing often involves significant time commitments for patients, including visits to labs and waiting 2 to 5 days for results. Now, a new TSH immunoassay performed on a silicon chip delivers results in approximately 30 minutes.
Hypothyroidism impacts about 10% of the U.S. population, making the Thyroid Stimulating Hormone (TSH) test the most frequently conducted immunoassay in the United States. Traditional testing often involves significant time commitments for patients, including visits to labs and waiting 2 to 5 days for results. Now, a new TSH immunoassay performed on a silicon chip delivers results in approximately 30 minutes.
Genalyte (San Diego, CA, USA) has received U.S. Food and Drug Administration (FDA) approval for its groundbreaking immunoassay, the first of its kind to be cleared for use on a silicon chip-based device. The Maverick Diagnostic System (MDS) incorporates silicon chip-based photonic ring resonator technology, enabling the execution of multiple rapid tests simultaneously from a small sample of whole blood or serum. This system is also connected to the cloud, facilitating the retrieval of assay protocols and enabling clinical oversight. Genalyte’s innovation effectively minimizes the traditional bulky lab machinery to 3% of its original size while still delivering lab-quality results.
The compact Maverick Diagnostic System, comparable in size to a small microwave and weighing just 30 pounds, offers lab-quality results on-site, representing a significant shift in laboratory processing much like the transformation brought by silicon chips in computing. This technology promises to significantly reduce the turnaround time for lab results by 98%, potentially making it available at point-of-care locations in the future. This FDA clearance not only marks a critical achievement for Genalyte but also for the diagnostic industry, confirming the company’s capability to deliver precise and rapid lab results.
“This TSH assay is a powerful tool to rapidly diagnose patients and get them on a path to treatment quicker,” said CEO of Genalyte Ashraf Hanna, Ph.D., M.D. “This technology is groundbreaking for immunoassay development which hasn’t changed conceptually since 1959. The Maverick has the potential to allow a broad menu of blood testing in a small, portable device at the point of care."
Source:
https://www.labmedica.com/molecular-diagnostics/articles/294802003/silicon-chip-based-blood-test-rapidly-diagnoses-patients-with-hypothyroidism.html
Read more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



