Roche’s Vabysmo Shows Sustained Improvements in Vision, Retinal Drying in Retinal Vein Occlusion
February 4, 2024
Source: drugdu
 332
332
Davy James
Vabysmo is the first bispecific antibody approved to treat ocular conditions such as diabetic macular edema and wet age-related macular degeneration.
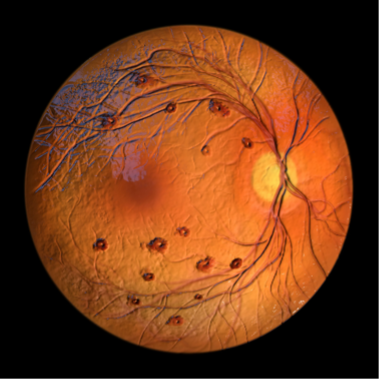 Findings from the global Phase III BALATON (NCT04740905) and COMINO (NCT04740931) trials show Roche’s Vabysmo (faricimab-svoa) produced sustained retinal drying and improved vision in patients with retinal vein occlusion (RVO).1 Vabysmo, the first bispecific antibody approved to treat ocular conditions, inhibits a pair of disease pathways involved in multiple vision-threatening retinal conditions by neutralizing angipoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A).2 In February 2022, Vabysmo became the first and only FDA-approved injectable eye medicine for diabetic macular edema and wet age-related macular degeneration.
Findings from the global Phase III BALATON (NCT04740905) and COMINO (NCT04740931) trials show Roche’s Vabysmo (faricimab-svoa) produced sustained retinal drying and improved vision in patients with retinal vein occlusion (RVO).1 Vabysmo, the first bispecific antibody approved to treat ocular conditions, inhibits a pair of disease pathways involved in multiple vision-threatening retinal conditions by neutralizing angipoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A).2 In February 2022, Vabysmo became the first and only FDA-approved injectable eye medicine for diabetic macular edema and wet age-related macular degeneration.
“This is the first time that vision and anatomical improvements have been maintained for more than a year in global Phase III studies for both branch and central retinal vein occlusion,” Levi Garraway, MD, PhD, Genentech chief medical officer and head of Global Product Development, said in a news release. “These long-term results build on the strong clinical and real-world data reinforcing Vabysmo as an effective treatment option for people affected by retinal conditions that can cause vision loss.”
The BALATON and COMINO trials were both randomized, multicenter, double-masked, global studies comparing the efficacy and safety of Vabysmo to aflibercept. BALATON enrolled 553 patients with branch retinal vein occlusion, whereas COMINO enrolled 729 patients with central retinal or hemiretinal vein occlusion.
In both trials, over the first 20 weeks, patients were randomly assigned 1:1 to receive monthly injections of Vabysmo 6.0 mg or aflibercept 2.0 mg over six months. After 24 weeks and up to 72 weeks, all patients in the trials were administered Vabysmo 6.0 mg up to every four months, as part of a treat-and-extend dosing regimen.
Both trials had a primary endpoint of change in best-corrected visual acuity from baseline at 24 weeks. Secondary endpoints for both trials from baseline to week 24 included change in central subfield thickness (CST) and retinal fluid drying. For weeks 24-72, the secondary endpoints were change in best-corrected visual acuity (BCVA) from baseline, change in CST from baseline, and proportion of patients on treat-and-extend intervals. Investigators observed that the improvements in vision and decreases in retinal fluid over the first 24 weeks continued up to 72 weeks.
In the BALATON trial, after 72 weeks, patients administered first-line Vabysmo gained 18.1 letters on the eye chart, whereas those who switched from aflibercept to Vabysmo gained 18.8 letters. Across the first 24 weeks, the vision gains in those administered Vabysmo were +16.8 eye chart letters compared with +17.5 letters in those administered aflibercept.
For retinal drying, at 72 weeks, patients administered first-line Vabysmo experienced a 310.9 µm decrease in retinal swelling as measured by CST, whereas patients who switched from aflibercept to Vabysmo experienced a 307 µm decrease in CST. Across the first 24 weeks of the trial, decreases in CST were 314.5 µm in patients administered Vabysmo compared with 307.6 µm in patients administered aflibercept.
At 72 weeks in the COMINO trial, patients administered first-line Vabysmo gained 16.9 eye chart letters, whereas those who switched from aflibercept to Vabysmo gained 17.1 eye chart letters. Over the trial’s first 24 weeks, vision gains were +16.9 eye chart letters in those administered Vabysmo compared with +17.3 letters in those administered aflibercept.
Patients administered first-line Vabysmo experienced a 465.9 µm decrease in retinal swelling in CST at 72 weeks compared with 460.6 µm in patients switched from aflibercept to Vabysmo. Over the first 24 weeks, decreases in CST were 462.3 µm in patients administered Vabysmo compared with 447.8 µm with aflibercept.
“The sustained vision improvements and retinal drying seen up to 72 weeks reaffirm Vabysmo as an effective treatment for retinal vein occlusion,” Ramin Tadayoni, MD, PhD, head of ophthalmology at Université Paris Cité in Paris and president of EURETINA, said in a press release. “More treatment options are needed to better serve people living with this condition, and these data show Vabysmo can potentially improve outcomes while reducing the number of clinic visits needed.”
By editorRead more on
- Gusekirumab Injection Accepted by CDE, Multiple Pipelines Advancing Simultaneously March 4, 2026
- Yifan Pharmaceutical’s teriparatide injection has been accepted by the CDE (Center for Drug Evaluation), adding a new domestic player to the osteoporosis treatment field March 4, 2026
- //news.yaozh.com/archive/47318.html PD-1 sales surge March 4, 2026
- A major breakthrough! Roche’s oral BTK inhibitor achieves its third Phase III clinical trial victory, a game-changer in the multi-billion dollar MS (manufactured pharmaceuticals) market. March 4, 2026
- GB19 Injection Approved for Clinical Trials of Cutaneous Lupus Erythematosus March 4, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



