Results from Clinical Trials of Two Henlius’ Novel Products Released at ASCO GI 2024
January 19, 2024
Source: drugdu
 494
494
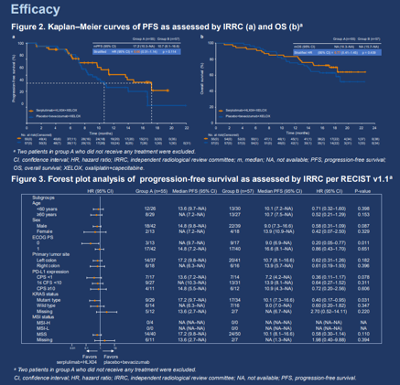 Recently, the latest clinical data of two Henlius products were released online and will be presented in poster sessions at the 2024 ASCO Gastrointestinal Cancers Symposium (ASCO GI), namely, the phase 2/3 study (HLX10-015-CRC301) of Henlius’ NMPA approved anti-PD-1 monoclonal antibody (mAb) HANSIZHUANG (serplulimab) in metastatic colorectal cancer (mCRC) with Professor Rui-Hua Xu of Sun Yat-Sen University Cancer Center as the leading principal investigator, and the phase 2 study (HLX22-GC-201) of Henlius’ novel anti-HER2 mAb, HLX22, combined with HANQUYOU (trastuzumab for injection, HLX02, trade name in Europe: Zercepac®; trade names in Australia: Tuzucip® and Trastucip®) and chemotherapy for the first-line treatment of HER2-positive gastric/gastroesophageal junction (G/GEJ) cancer with Professor Jin Li of Shanghai East Hospital, School of Medicine, Tongji University as the leading principal investigator of this study.
Recently, the latest clinical data of two Henlius products were released online and will be presented in poster sessions at the 2024 ASCO Gastrointestinal Cancers Symposium (ASCO GI), namely, the phase 2/3 study (HLX10-015-CRC301) of Henlius’ NMPA approved anti-PD-1 monoclonal antibody (mAb) HANSIZHUANG (serplulimab) in metastatic colorectal cancer (mCRC) with Professor Rui-Hua Xu of Sun Yat-Sen University Cancer Center as the leading principal investigator, and the phase 2 study (HLX22-GC-201) of Henlius’ novel anti-HER2 mAb, HLX22, combined with HANQUYOU (trastuzumab for injection, HLX02, trade name in Europe: Zercepac®; trade names in Australia: Tuzucip® and Trastucip®) and chemotherapy for the first-line treatment of HER2-positive gastric/gastroesophageal junction (G/GEJ) cancer with Professor Jin Li of Shanghai East Hospital, School of Medicine, Tongji University as the leading principal investigator of this study.
Colorectal cancer (CRC) is one of the most common malignancies globally. Over 1.9 million newly diagnosed cases and more than 900,000 deaths were estimated in 2020[1]. The standard of care for metastatic CRC (mCRC) involves the combination of vascular endothelial growth factor (VEGF) inhibitor, such as bevacizumab, and systemic chemotherapy[2-4]. Previously, HANSIZHUANG was approved in China for the treatment of MSI-H solid tumours in March 2022 based on its excellent efficacy data, and then was recommended by the CSCO Guidelines for Colorectal Cancer and the CSCO Clinical Practice Guidelines on Immune Checkpoint Inhibitor. The company is continuing to explore immuno-oncology therapy for this arena, with the goal of delivering more effective treatment for a broader population of patients. The results of this study demonstrated that serplulimab plus HLX04 (a bevacizumab biosimilar) and XELOX markedly prolonged PFS compared to placebo plus bevacizumab and XELOX, with a manageable safety profile.
Gastric/gastroesophageal junction (G/GEJ) cancer represents a global healthcare challenge with more than 1 million new cases estimated in 2020[1]. G/GEJ cancer is often diagnosed at the advanced stage, and the prognosis of advanced G/GEJ cancer is poor, with a 5-year relative survival rate of only 6%[5,6]. And the prognosis for patients with HER2-positive disease used to be even worse than those with HER2-negative disease[5-7]. HLX22 is an innovative anti-HER2 mAb that was introduced from AbClon, Inc. and further researched and developed by Henlius. HLX22 can bind to HER2 subdomain IV at a different binding site from trastuzumab, which allows the simultaneous binding of HLX22 and trastuzumab to HER2. The pre-clinical studies showed that the combination therapy of HLX22 and trastuzumab would inhibit the cell proliferation induced by epidermal growth factor (EGF) and Histidine-Rich Glycoprotein 1 (HRG1) and enhance the antitumor activity in vitro and in vivo. The phase 1 clinical trial of HLX22 demonstrates that HLX22 is well tolerated and has good safety profiles. As of now, no similar dual HER2 blockade therapy for the treatment of HER2-positive gastric cancer has received approval for commercialization globally.
https://mp.weixin.qq.com/s/5OO235MFVv0Yp4cywoL2UQ
By editorRead more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



