Recombinant Human Anti-Tetanus Toxin Monoclonal Antibody Injection by SINOPHARM Lanzhou Institute of Biological Products Receives Clinical Trial Approval
July 25, 2024
Source: drugdu
 371
371
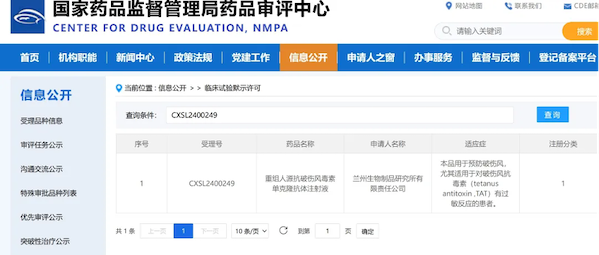 On July 12, Recombinant Human Anti-Tetanus Toxin Monoclonal Antibody Injection, a Class I new drug independently developed by SINOPHARM Lanzhou Institute of Biological Products, was granted a clinical trial approval notice by the State Drug Administration.
On July 12, Recombinant Human Anti-Tetanus Toxin Monoclonal Antibody Injection, a Class I new drug independently developed by SINOPHARM Lanzhou Institute of Biological Products, was granted a clinical trial approval notice by the State Drug Administration.
Tetanus is a persistent tonic contraction and paroxysmal spasm of skeletal muscles throughout the body caused by the invasion of Clostridium tetani through a break in the skin or mucous membrane. Globally, the morbidity and mortality rate of tetanus is 30% to 50% after onset of the disease, even with aggressive treatment; in the absence of medical intervention, the mortality rate approaches 100%. According to the World Health Organization, tetanus currently kills about 1 million people worldwide each year.
The term "tetanus shot" is short for tetanus passive immunization agent, which is usually isolated from healthy human or horse blood that has been vaccinated against tetanus. Tetanus injections are often needed in cases of unclean or contaminated wounds to treat or prevent diseases caused by tetanus. Since the incubation period of tetanus onset is 7-8 days on average, and the onset of the disease is 24 hours at the earliest, the earlier the tetanus injection is given after the injury, the better.
The new type of tetanus injection developed by Lanzhou Institute of Biological Products is a fully human monoclonal antibody injection, which is obtained through genetic engineering technology, with high purity and low impurity, and is easy to be mass-produced. At present, there is no similar monoclonal antibody on the market worldwide.
In recent years, Lanzhou Institute of Biological Products has been striving to build a platform for recombinant protein drug development technology, combining its own advantages, focusing on differentiated development, and actively laying out on recombinant protein products. The clinical license of recombinant human anti-tetanus toxin monoclonal antibody injection is another achievement of the recombinant protein drug development platform of Lanzhou Institute of Biological Products, which further improves the platform construction. Meanwhile, the successful development of this product will further enrich the types of "tetanus injections" and provide new, safer, more efficient and accessible products for the society.
https://mp.weixin.qq.com/s/Vcp2emo9KPhEOTUqhVZFqA
By editorRead more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



