Non-Endoscopic Capsule Sponge Device Helps Detect Esophageal Cancer
February 8, 2024
Source: drugdu
 360
360
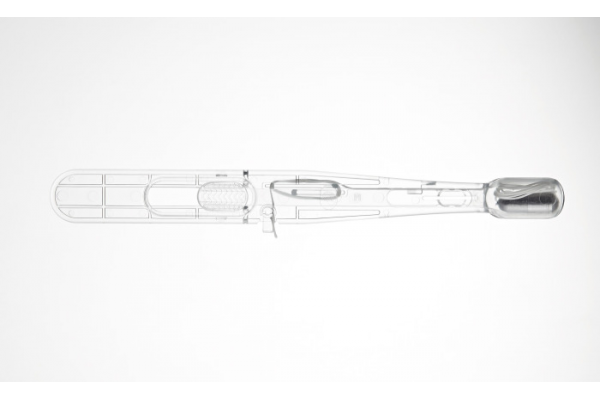
Barrett's esophagus is a condition often resulting from reflux, characterized by stomach acid damaging the esophagus lining and causing cell changes. While these cells aren't initially cancerous, there's a risk they might transform into esophageal cancer, a type where cells in the esophagus proliferate uncontrollably, potentially spreading to other body parts. Esophageal cancer is among the deadliest cancers in adults, and early detection significantly improves survival rates compared to a diagnosis at an advanced stage. Since the symptoms of esophageal cancer can mimic heartburn and reflux, conducting early tests for cancer detection is crucial. Now, a non-endoscopic capsule sponge device has been designed to collect pan-esophageal samples which are then sent for laboratory testing to detect esophageal pre-cancer and other conditions.
Cyted’s (Cambridge, UK) EndoSign cell collection device is designed to detect and monitor conditions such as chronic reflux and Barrett’s esophagus, ultimately aiming to prevent esophageal adenocarcinoma. The EndoSign capsule sponge is comprised of an applicator containing a small capsule, about the size of a vitamin pill. Inside the capsule is a sponge linked to a pre-bunched surgical thread, simplifying the administration process. The patient swallows the capsule and thread with water. Once the capsule dissolves in the stomach, typically in about 7 minutes, it releases the sponge.
As the sponge is gently and steadily retracted using the thread, it gathers cells along the entire esophagus. After retrieval, the sponge is placed in a storage container provided in the cell preservation kit and sent to Cyted for biomarker analysis. EndoSign offers a comprehensive diagnostic service, encompassing cell collection, sample preservation, analysis, and reporting. The entire procedure can be completed in under 10 minutes and usually doesn’t require sedation, though some patients might receive a local anesthetic spray to minimize discomfort. EndoSign technology has already shown significant potential in various digestive tract applications. Cyted has recently achieved 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its EndoSign cell collection device.
“This clearance opens up significant opportunities for Cyted across new geographies and health systems and confirms our device is safe and effective for use in the US.,” said Marcel Gehrung, CEO and Co-founder of Cyted “Combined with novel biomarkers, Cyted’s potential to transform the way patients with chronic reflux are identified and monitored is significant and this clearance is a major step for our expansion.”
Source:
By editorRead more on
- Gusekirumab Injection Accepted by CDE, Multiple Pipelines Advancing Simultaneously March 4, 2026
- Yifan Pharmaceutical’s teriparatide injection has been accepted by the CDE (Center for Drug Evaluation), adding a new domestic player to the osteoporosis treatment field March 4, 2026
- //news.yaozh.com/archive/47318.html PD-1 sales surge March 4, 2026
- A major breakthrough! Roche’s oral BTK inhibitor achieves its third Phase III clinical trial victory, a game-changer in the multi-billion dollar MS (manufactured pharmaceuticals) market. March 4, 2026
- GB19 Injection Approved for Clinical Trials of Cutaneous Lupus Erythematosus March 4, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



