Rubicon WIPI Axis Regulates Extracellular Secretion Synthesis during Aging Process
September 20, 2024
Source: drugdu
 494
494
picture
Writing | Sister Xian
Extracellular vesicles are important mediators of intercellular communication, consisting of small vesicles with diameters ranging from 30-150 nanometers. They can transport biomolecules such as proteins, lipids, and nucleic acids from one cell to another, thereby regulating the behavior of recipient cells. Due to their roles in various physiological and pathological processes, extracellular vesicles have become a hot topic in biomedical research. Studies have found that as age increases, the number and composition of circulating extracellular vesicles change, which may have significant impacts on the aging process. The changes in miRNA and other molecules in extracellular vesicles with age may affect cell function and tissue regeneration ability [1]. However, how extracellular vesicles change during aging and the molecular mechanisms behind these changes are currently not fully understood.
The formation of extracellular vesicles is a complex multi-step process involving the endosomal pathway and the biological formation of multivesicular vesicles (MVBs). Intraluminal vesicles (ILVs) sprout inward through the endosomal membrane to form MVBs, which then fuse with the plasma membrane to release ILVs as extracellular vesicles [2]. The endocrine sorting complex required for transport (ESCRT) is responsible for sorting membrane proteins and certain cargo molecules into MVBs within cells, and then participating in regulating the degradation of these cargo molecules or their release as extracellular vesicles. Research has shown that ESCRT and its related proteins can be recruited to specific sites in endosomes to mediate the formation of ILVs [3], but the detailed mechanism of this recruitment in exosome biosynthesis remains largely elusive. Autophagy is a process of degradation and circulation of substances within cells, which is crucial for maintaining cellular homeostasis. During autophagy, double membrane organelles called autophagosomes isolate cytoplasmic components and fuse with lysosomes to degrade their contents. This process is coordinated by a group of autophagy related (ATG) genes and is negatively regulated by the Rubicon, Run domain beclin-1-interacting and cysteine rich domain containing proteins that interact with Beclin-1. Although studies have shown that the formation of extracellular vesicles is closely related to autophagy, the specific mechanism is not yet fully understood, and determining whether the biosynthesis of extracellular vesicles requires the entire autophagy mechanism or some specific autophagy related factors remains a complex issue.
Autophagy is a process of degradation and circulation of substances within cells, which is crucial for maintaining cellular homeostasis. During autophagy, double membrane organelles called autophagosomes isolate cytoplasmic components and fuse with lysosomes to degrade their contents. This process is coordinated by a group of autophagy related (ATG) genes and is negatively regulated by the Rubicon, Run domain beclin-1-interacting and cysteine rich domain containing proteins that interact with Beclin-1. Although studies have shown that the formation of extracellular vesicles is closely related to autophagy, the specific mechanism is not yet fully understood, and determining whether the biosynthesis of extracellular vesicles requires the entire autophagy mechanism or some specific autophagy related factors remains a complex issue.
Recently, Tamotsu Yoshimori's team from Osaka University in Japan published an article titled "The Rubicon WIPI axis regulates exosome biogenesis during aging" online in Nature Cell Biology. They conducted a comprehensive RNA interference screening of autophagy related factors and found that the autophagy negative regulator Rubicon is crucial for exosome release and can actively regulate exosome biosynthesis in a way unrelated to autophagy. It has been confirmed that Rubicon mediates the intracellular recruitment of WD repeat domain phosphoinosine interacting protein 2 (WIPI2), which can interact with ESCRT required for MVB formation. It was also found that Rubicon is crucial for age-related exosome secretion of miRNAs associated with cellular aging and aging related pathways.
In order to determine whether the release of extracellular vesicles requires the entire autophagy pathway or only specific autophagy factors, the researchers in this study first performed RNA interference (RNAi) screening on autophagy related factors, knocking down the ATG gene and RUBCN in human mesenchymal stem cells (hMSCs), and isolated extracellular vesicles from the culture medium for western blot detection. The results showed that extracellular vesicle secretion did not require the entire autophagy pathway, but only certain autophagy factors. More importantly, researchers found that Rubicon is localized to CD63+endosomes, and endosome PI3P can play a role in the formation of MVB in a Rubicon dependent manner. The expression level of Rubicon is the determining factor of exosome secretion, and its regulation of exosome secretion does not rely on autophagy, but rather on a previously unknown pathway.
In order to investigate how Rubicon regulates exosome secretion, researchers subsequently explored its interacting proteins during exosome biosynthesis and found that WIPI2 can interact with Rubicon to promote exosome secretion. WIPI2 is a member of the WIPI family of proteins, classified as a β - propeller structure capable of binding to inositol polyphosphate (PROPPIN). Therefore, as an effector protein that binds to PI3P, it is crucial for the formation of autophagosomes. However, its role in exosome secretion remains largely unexplored. So how does WIPI2 synergistically regulate exosome secretion with Rubicon? The research results showed that Rubicon recruits specific WIPI proteins into endosomes to promote exosome secretion by interacting with WIPI proteins through its helix curl region rich protein domain (HCR). Only WIPI proteins specifically recruited into Rubicon expressed endosomes can promote exosome biosynthesis. In the absence of Rubicon, overexpression of WIPI2 does not increase exosome secretion. Meanwhile, the research findings indicate that the Rubicon WIPI axis is crucial for the formation of MVB. Through interaction group analysis, researchers found that WIPI2d can interact with ESCRT components, and these interacting ESCRT proteins are crucial for the formation of exosomes. Further experimental results confirmed that Rubicon promotes the recruitment of WIPI2 into the endosome, and then WIPI2 recruits ESCRT protein into the endosome to form ILV (Figure 1).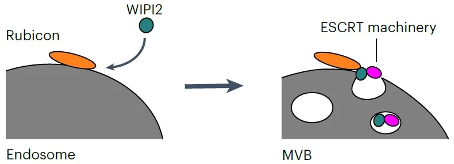 In order to investigate the role of Rubicon WIPI axis in vivo, researchers also evaluated the secretion of extracellular vesicles in Rubicon knockout (KO) mice. The results showed that the number of small extracellular vesicles in the serum of Rubicon KO mice was significantly reduced, but the number of large extracellular vesicles did not decrease. Adipose derived mesenchymal stem cells (AD-MSC, the main source of extracellular vesicles) showed a decrease in extracellular vesicle production in Rubicon KO mice. At the same time, researchers also found that an increase in the release of extracellular vesicles by Rubicon is necessary during the aging process of mice. As age increases, Rubicon gradually accumulates and promotes the secretion of extracellular vesicles in mice. Further small RNA sequencing revealed that Rubicon is necessary for age-related changes in the extracellular vesicle miRNA profile, which are associated with aging related signaling pathways. Taking miR-26a-5p and miR-486a-5p as examples, research has shown that they are secreted into serum exosomes in a Rubicon dependent manner during the aging process, inhibiting the expression of aging related genes and promoting cellular aging. In addition, by adding the conditioned medium of aging donor cells to recipient cells, researchers confirmed that Rubicon mediated cell aging through exosome secretion rather than autophagy pathway.
In order to investigate the role of Rubicon WIPI axis in vivo, researchers also evaluated the secretion of extracellular vesicles in Rubicon knockout (KO) mice. The results showed that the number of small extracellular vesicles in the serum of Rubicon KO mice was significantly reduced, but the number of large extracellular vesicles did not decrease. Adipose derived mesenchymal stem cells (AD-MSC, the main source of extracellular vesicles) showed a decrease in extracellular vesicle production in Rubicon KO mice. At the same time, researchers also found that an increase in the release of extracellular vesicles by Rubicon is necessary during the aging process of mice. As age increases, Rubicon gradually accumulates and promotes the secretion of extracellular vesicles in mice. Further small RNA sequencing revealed that Rubicon is necessary for age-related changes in the extracellular vesicle miRNA profile, which are associated with aging related signaling pathways. Taking miR-26a-5p and miR-486a-5p as examples, research has shown that they are secreted into serum exosomes in a Rubicon dependent manner during the aging process, inhibiting the expression of aging related genes and promoting cellular aging. In addition, by adding the conditioned medium of aging donor cells to recipient cells, researchers confirmed that Rubicon mediated cell aging through exosome secretion rather than autophagy pathway.
In summary, this study reveals the key role of the Rubicon WIPI axis in exosome biosynthesis, which can positively regulate ESCRT dependent exosome secretion, and points out the importance of this axis in regulating changes in the quantity and quality of extracellular vesicle miRNAs during aging. These findings not only enhance our understanding of the biosynthetic mechanisms of extracellular vesicles, but also provide new targets for developing therapeutic strategies targeting age-related diseases.
By editorRead more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



