MMI receives de novo nod for microsurgery robot
April 15, 2024
Source: drugdu
 329
329
Dive Brief
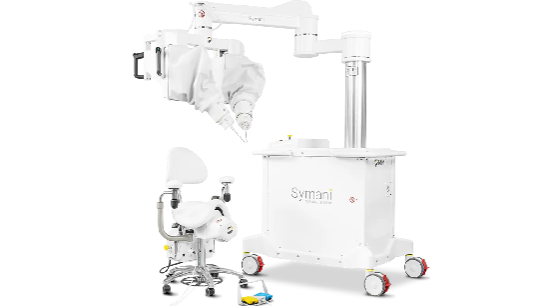
Medical Microinstruments (MMI) has received de novo authorization for a robotic system that enables surgeons to reconnect tiny blood vessels, the company said Monday.
The Food and Drug Administration authorized the device, called the Symani Surgical System, for soft tissue manipulation to perform microsurgery, a way to restore blood flow and redirect fluid during reconstruction or repair.
CEO Mark Toland said the commercial availability of Symani could increase the number of physicians who can perform complicated microsurgical procedures. MMI plans to immediately launch the system in the U.S.
Dive Insight
Microsurgery and supermicrosurgery, a technique for 0.3mm to 0.8mm vessels, are microscope-assisted operations that enable physicians to take a piece of tissue from one part of the body and use it to reconstruct another area. By reconnecting blood vessels, surgeons can restore form and function to parts of the body damaged by trauma or disease.
The potential for robotics to improve the intricate procedures has spurred the development of systems such as MMI’s Symani and Microsure’s Musa. Microsure called Musa the world’s first surgical robot for open microsurgery when it received a CE mark in 2019. The company is targeting FDA clearance.
MMI claimed its own first Monday, when it said the de novo authorization made Symani the only commercially available platform in the U.S. for reconstructive microsurgery. Surgeons can use the device in procedures such as post-mastectomy breast cancer reconstruction, extremity reconstruction using free tissue transfer and lymphatic system repair.
Toland said in a statement that the “U.S. is facing a potentially dire shortage of physicians, and that shortage acutely impacts specialized fields of medicine, such as microsurgery.” The MMI CEO sees the de novo authorization as a way to help hospitals grow their open surgical programs and expand the pool of physicians who can perform the procedures.
Other goals include increasing patient access “to the most advanced techniques for surgeries in complex disease states, such as lymphedema,” Toland said, while challenging surgeons’ “definitions of ‘treatable’ and ‘untreatable’ and empowering them to solve cases that have historically been too difficult to treat.”
In Europe, physicians have used the system for free flap surgery in cranio- and maxillofacial procedures, extremity reconstruction and lymphatic surgery, all in a limited number of surgeries. Early users discussed the promise of the technology, while noting that procedures typically took longer using the robot and cautioning that more data is needed.
Source: https://www.medtechdive.com/news/mmi-symani-robot-microsurgery-fda/712632/
Read more on
- Gusekirumab Injection Accepted by CDE, Multiple Pipelines Advancing Simultaneously March 4, 2026
- Yifan Pharmaceutical’s teriparatide injection has been accepted by the CDE (Center for Drug Evaluation), adding a new domestic player to the osteoporosis treatment field March 4, 2026
- //news.yaozh.com/archive/47318.html PD-1 sales surge March 4, 2026
- A major breakthrough! Roche’s oral BTK inhibitor achieves its third Phase III clinical trial victory, a game-changer in the multi-billion dollar MS (manufactured pharmaceuticals) market. March 4, 2026
- GB19 Injection Approved for Clinical Trials of Cutaneous Lupus Erythematosus March 4, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



