Medtronic to chase Boston Scientific, Abbott in left atrial appendage closure market
November 30, 2023
Source: drugdu
 404
404
 Dive Brief
Dive Brief
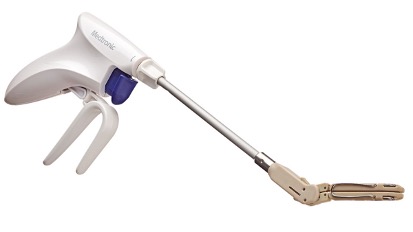
Medtronic said Monday it is launching a device in the U.S. designed to close the left atrial appendage (LAA) of the heart in patients undergoing cardiac surgery. The treatment is intended for patients who have atrial fibrillation (AFib), an arrhythmia that can lead to stroke.
The introduction of the implantable LAA clip, called Penditure, marks Medtronic’s entry into the fast-growing market for left atrial appendage closure devices, led by Boston Scientific’s Watchman franchise.
Medtronic said it acquired the LAA exclusion system in August from Miami-based medical device incubator Syntheon, in a move to expand its cardiac surgery portfolio. Terms of the transaction were not disclosed.
Dive Insight
Medtronic’s device is the newest challenger to Boston Scientific’s Watchman in a growing market that includes Abbott’s Amplatzer Amulet treatment, which gained approval from the Food and Drug Administration in 2021, and AtriCure, whose AtriClip device was the first LAA exclusion system to get FDA’s nod, in 2010.
Watchman has been a top growth driver for Boston Scientific, with sales climbing 23% in the third quarter. The company has pegged the current market for left atrial appendage closure at $1.4 billion and predicts that number could quadruple to more than $6 billion by 2030. Boston Scientific won FDA approval for the latest generation of its device, Watchman FLX Pro, in September.
Atrial fibrillation increases the risk for stroke, causing about one in seven cases, according to the Centers for Disease Control and Prevention. Most stroke-causing blood clots originate in the LAA.
“We estimate the left atrial appendage procedure market in the U.S. to be about 150 million patients. We will be very competitive to treat these patients with a differentiated surgical LAA solution,” Karim Bandali, president of Medtronic’s cardiac surgery business, said in an emailed statement.
The left atrial appendage closure procedure seals the opening to the heart’s LAA to keep clots from entering the bloodstream and causing strokes in people with AFib. Guidelines from the American College of Cardiology, American Heart Association and Society of Thoracic Surgeons recommend that patients with AFib who are undergoing a related cardiac surgery procedure should have their LAA closed.
Medtronic said its new offering received 510(k) clearance from the FDA in August and is commercially available in the U.S. on a limited basis. The device is the only LAA clip that can be repositioned after deployment during a procedure, according to the company.
“While we hope we don’t need to use it often, the Penditure clip is recapturable and redeployable should we ever want to reposition an already deployed clip, which is a nice safety feature,” Gorav Ailawadi, professor of cardiac surgery at the University of Michigan, said in a statement from Medtronic.
The medtech company plans to begin enrolling patients in a post-market study of the device in early 2024. The CLIP-IT study will be a single-arm, nonrandomized trial evaluating about 150 patients at 25 U.S. sites.
Source:
https://www.medtechdive.com/news/medtronic-boston-scientific-abbott-left-atrial-appendage-closu/700802/
Read more on
- Anglikang Adenosylcobalamin Capsules Obtain Drug Registration Certificate March 6, 2026
- Two Minoxidil Topical Solution Applications from its Subsidiary Approved March 6, 2026
- Zhejiang Medicine’s ARX305 Initiates Phase II Clinical Trial March 6, 2026
- Indacaterol Mometasone Inhalation Powder Clinical Trial Approved for Asthma Treatment March 6, 2026
- Harsco Pharmaceutical’s innovative drug HSK50042 tablets receive clinical approval for a new indication March 6, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



