Kunyao Group’s Class 1 new drug is coming! Seize the market worth 210 billion yuan
November 26, 2024
Source: drugdu
 698
698
Recently, the CDE official website showed that KPC000154 tablets, a Class 1 new drug declared by Kunming Pharmaceutical Group, have obtained implied approval for clinical trials, with indications for non-alcoholic fatty liver disease. According to data from MineNet, the sales of digestive system and metabolic drugs (chemical+biological) in China's three major terminal markets and six major markets (detailed statistical scope at the end of this article) will exceed 210 billion yuan in 2023.
Reference No. Drug name Applicant name Indication Registration classification
CXHL2400923 KPC000154 pills KPC (Hengqin, Zhuhai) Technology Co., Ltd; KPC Pharmaceuticals Inc. NASH 1
CXGL2400922 KPC000154 pills KPC (Hengqin, Zhuhai) Technology Co., Ltd; KPC Pharmaceuticals Inc. NASH 1
KPC000154 tablets are a Class 1 innovative drug independently developed by Kunming Pharmaceutical Group. Its clinical application was accepted by CDE in September 2024 and was recently approved for clinical use in the treatment of non-alcoholic fatty liver disease.
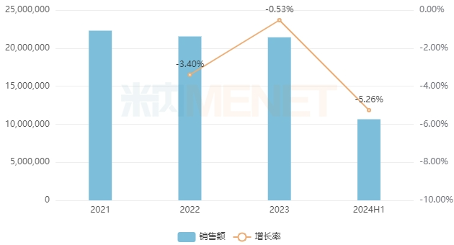
According to data from MineNet, the sales of digestive systems and metabolic drugs (chemical+biological) in China's three major terminal markets and six major markets will remain above 210 billion yuan from 2021 to 2023, but will continue to decline due to the impact of price reductions in centralized procurement. From the distribution of subclasses in the first half of 2024, drugs for diabetes accounted for more than 30%, drugs, vitamins and mineral supplements for diseases related to gastric acid secretion accounted for more than 10%, and drugs for bile/liver diseases accounted for more than 9%.
In recent years, sales of digestive system and metabolic drugs (chemical+biological) in China's three major terminal markets and six major markets (unit: 10000 yuan)
 Non alcoholic steatohepatitis is a type of fatty liver disease with a complex pathogenesis, and there are currently unmet clinical needs. Among the domestically developed new drugs for this indication (including those introduced by domestic enterprises), Lanifibranor tablets from Zhengda Tianqing Pharmaceutical/INVentiva are in phase III clinical trials, HSK31679 tablets from Hisilicon, HEC88473 injection and HEC96719 tablets from Dongyangyao, ZSP1601 tablets from Zhongsheng Pharmaceutical, recombinant human FGF21 Fc fusion protein for injection from Anyuan Pharmaceutical, and ASC41 tablets from Ganlai Pharmaceutical are in phase II clinical trials.
Non alcoholic steatohepatitis is a type of fatty liver disease with a complex pathogenesis, and there are currently unmet clinical needs. Among the domestically developed new drugs for this indication (including those introduced by domestic enterprises), Lanifibranor tablets from Zhengda Tianqing Pharmaceutical/INVentiva are in phase III clinical trials, HSK31679 tablets from Hisilicon, HEC88473 injection and HEC96719 tablets from Dongyangyao, ZSP1601 tablets from Zhongsheng Pharmaceutical, recombinant human FGF21 Fc fusion protein for injection from Anyuan Pharmaceutical, and ASC41 tablets from Ganlai Pharmaceutical are in phase II clinical trials.
Kunyao Group was established in 1951 and is a unique integrated enterprise in the research and development, production, and marketing of natural plant-based medicines in China. At present, the company has more than 600 product approval numbers, covering various dosage forms such as powder injections, injections, capsules, tablets, granules, pills, and powders. The core products include Kunyao Xuesaitong series, artemether series, Kunyao Canling Jianpi Stomach Granules, Shugan Granules, Xiangsha Pingwei Granules, Beck Norton Afacalcitriol Soft Capsules, Sodium Hyaluronate Injection, etc.
In recent years, Kunyao Group has been driven by research and development innovation, continuously increasing R&D investment around the company's core areas and enriching its product pipeline. According to the annual report data, Kunming Pharmaceutical Group invested 125 million yuan in research and development in 2023, a year-on-year increase of 2.8%; The R&D expenses for the first three quarters of 2024 increased by over 38% year-on-year.
At present, Kunyao Group is developing new drugs, and the injection KPCXM18 (KYAZ01-2011-020), a Class 1 traditional Chinese medicine/natural medicine suitable for ischemic stroke, has entered Phase II clinical trials. Its research data is currently being statistically analyzed; The new drug KY100001, a Class 1 chemical drug, is currently in Phase I clinical trials. It is an IDH1 selective inhibitor, and there are currently no domestically produced new drugs with the same target approved in China.
Kunyao Group is partially researching new drugs
Drug name Drug type Registration classification Latest study progress Indication
KPCXM18 for injection Chinese patent drug Class 1 of New drug Phase II study Acute ischemic stroke
KY100001 pills Chemical drug Class 1 of New drug Phase I study Late stage solid tumors with IDH1 genetic mutations
Denosumab Injection Therapeutic biological product Class 2 of New drug Phase I study Osteoporosis in postmenopausal women at high risk of fractures
KPC000154 pills Chemical drug Class 1 of New drug IND approved NSAH
KPC-149 oral solution Chemical drug Class 2.2 of New drug IND approved FMF
In terms of generic drugs, since the beginning of this year, Kunyao Group's Clonazepam Injection and Colchicine Tablets have successfully passed the evaluation, both of which are the first in China to pass the evaluation. In addition, the carbonated sevelamer dry suspension has been newly registered and classified for production review, and currently only two domestic enterprises have the production approval for this product.
Read more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



