Johnson & Johnson’s autoimmune drug approved for new indication
May 14, 2025
Source: drugdu
 417
417
On May 9, Johnson & Johnson announced that its blockbuster drug Guselkumab was approved by the National Medical Products Administration for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, loss of response, or intolerance to traditional treatments or biologics.
This event marks the official entry of interleukin 23 (IL-23) targeted era into the treatment of ulcerative colitis in China.
1. China's first
As the world's first approved fully humanized IL-23 inhibitor, guselkumab (trade name: Tenoyada) precisely regulates the immune response through a unique dual mechanism of action: it not only directly binds to the IL-23 cytokine, but also targets IL-23-enriched CD64+ inflammatory cells, blocking this core pathogenic factor that drives immune-mediated diseases such as ulcerative colitis (UC) from the source.
Today, guselkumab was officially approved by the China National Medical Products Administration for the treatment of adult patients with moderate to severe active ulcerative colitis, becoming the first interleukin-23 inhibitor for the treatment of ulcerative colitis in China. In February of this year, guselkumab was approved for the treatment of Crohn's disease in the country. So far, the drug's approved indications in China have covered psoriasis, Crohn's disease and ulcerative colitis, providing extensive coverage of immune diseases related to the IL-23 pathway.
The approval is based on the pivotal Phase 2b/3 QUASAR study, which demonstrated the following therapeutic advantages for patients with UC who have not responded well to conventional treatments, other biologics, or JAK inhibitors:
Significant relief of symptoms such as diarrhea and bloody stools was observed in the first week of treatment, and the efficacy continued to increase with 12 weeks of induction treatment.
In the maintenance treatment phase at week 44, 50% of patients in the guselkumab 200 mg every 4 weeks group achieved the primary endpoint of clinical remission at week 44, and 45% of patients in the 100 mg every 8 weeks group achieved the same clinical remission, both significantly higher than the 19% in the placebo group (<0.001);
Endoscopic evaluation showed that 34% and 35% of patients in the 200 mg and 100 mg groups, respectively, achieved complete healing of the intestinal mucosa (MES=0), while only 15% of the placebo group achieved endoscopic remission (<0.001).
The safety advantage is outstanding: the most common adverse reaction (>2%) is respiratory tract infection, and the incidence of this adverse reaction is higher in the placebo group. In the maintenance study, the most common adverse reactions (>3%) were injection site reactions, arthralgia, and upper respiratory tract infection.
2. Reconstruction of the structure
Ulcerative colitis (UC) is a form of inflammatory bowel disease (IBD) that causes chronic inflammation of the intestine and damage to the colon lining. Currently, there is no cure for ulcerative colitis.
Traditional treatments for ulcerative colitis (UC) and Crohn's disease (CD) rely on anti-inflammatory drugs, glucocorticoids, and immunosuppressants, but all have significant limitations. Although aminosalicylic acid drugs (such as 5-ASA) can relieve mild inflammation, they cannot effectively induce remission of active CD or prevent recurrence of inactive CD; glucocorticoids have high short-term efficacy, but some patients become dependent or relapse within a year; immunosuppressants are limited in clinical application due to their non-specific mechanism of action, resulting in short-term toxicity and long-term health risks.
The advent of biological targeted drugs has ushered in the era of precision treatment. Currently, UC/CD biological drugs approved in China are divided into three categories:
TNF-α inhibitors: inhibit inflammation by blocking TNF receptor binding, but some patients develop drug resistance after taking the drugs and need to change the regimen frequently;
Integrin α4β7 inhibitors: bind to the surface of white blood cells, preventing them from passing through tissue layers and exacerbating inflammation, but there is a potential risk of inducing progressive multifocal leukoencephalopathy, and the safety is controversial;
IL-12/IL-23 inhibitors (such as ustekinumab): specifically target the Th1/Th17 immune pathway, while maintaining a high clinical remission rate, they exhibit strong safety and have the dual advantages of "high efficacy + low safety risk".
The total number of UC and CD patients in China increased from 490,000 in 2018 to 670,000 in 2022, and is expected to increase to 1.154 million in 2030. In recent years, China's UC/CD drug market has grown rapidly, from US$594 million in 2018 to US$1.051 billion in 2022, and is expected to reach US$5.49 billion in 2030. In 2022, biological drugs accounted for 13.7% of China's UC/CD drug market, and it is estimated that this will increase to 55.9% in 2030.
3. Johnson & Johnson's self-immunization layout
The autoimmune business is the core pillar of Johnson & Johnson's pharmaceutical sector, with revenue of US$17.828 billion in 2024, accounting for 31.3% of the pharmaceutical business. Although the sales of the major product Stelara (ustekinumab) fell by 4.6% to US$10.361 billion due to the impact of biosimilars, the company continued to consolidate its leading position in the industry through the strategy of "new and old product relay + cutting-edge target mergers and acquisitions".
Johnson & Johnson has built a product portfolio covering multiple indications such as psoriasis, Crohn's disease (CD), and ulcerative colitis (UC), with the IL-23/IL-12 pathway as the core.
The flagship product Stelara (ustekinumab) is the world's first IL-12/IL-23 dual-target inhibitor. Since its launch in 2009, its cumulative sales have exceeded US$100 billion. In 2023, its sales exceeded US$10 billion for the first time (US$10.858 billion). However, due to the expiration of patents and the impact of biosimilars, its sales in 2024 fell by 4.6% year-on-year to US$10.361 billion. However, Johnson & Johnson has extended its life cycle by expanding its subcutaneous injection dosage form and pediatric indications.
To cope with the patent cliff, Johnson & Johnson launched the second-generation IL-23 inhibitor Tremfya (guselkumab), which achieves a differentiated breakthrough by virtue of its dual mechanism of action (blocking IL-23 and regulating the activity of immune cells). In 2024, it was approved for the indications of Crohn's disease and ulcerative colitis, and became the world's first drug to defeat ustekinumab head-to-head in the CD indication. In 2024, Tremfya sales increased by 16.6% year-on-year to US$3.67 billion, and in Q1 2025, sales reached US$956 million, a significant year-on-year increase of 18.2%. If it maintains a compound growth rate of 15%-20%, it is expected to exceed US$6 billion in 2026 and become the core growth engine of Johnson & Johnson's autoimmune sector.
In order to cope with the fierce competition in mature tracks such as IL-23 and JAK, Johnson & Johnson has deployed differentiated targets through mergers and acquisitions, introduction and other strategies, and expanded to new targets such as TSLP, FcRn, STAT6, covering atopic dermatitis, myasthenia gravis and other broader indications. At the same time, we will make use of technological innovations such as bispecific antibodies and oral peptides to make our layout.
To address the pain point of compliance with biologics, Johnson & Johnson is accelerating the development of Icotrokinra (JNJ-2113), the world's first oral IL-23R antagonist. In the Phase 3 psoriasis trial, Icotrokinra achieved a 16-week PASI 90 response rate of 49.6%, significantly better than BMS's TYK2 inhibitor Sotyktu (36.8%). It is expected to submit its psoriasis marketing application in 2025, and its peak sales may exceed US$5 billion.
Johnson & Johnson's FcRn monoclonal antibody Nipocalimab was launched in the United States in April 2025 for the treatment of myasthenia gravis. The drug achieved the primary endpoint in the Phase 2 DALLIAS trial for Sjögren's syndrome. Its peak sales are expected to exceed US$5 billion, and it is expected to become a blockbuster product of the new generation of FcRn inhibitors after Efgartigimod.
In the field of bispecific antibodies, Johnson & Johnson acquired Proteologix for US$850 million in 2024, and obtained the IL-13/TSLP bispecific antibody PX128 (for atopic dermatitis and asthma) and the IL-13/IL-22 bispecific antibody PX130 (for atopic dermatitis). PX128 showed the potential to reduce the frequency of dosing in Phase 1 clinical trials by simultaneously inhibiting the IL-13 and TSLP pathways, and is expected to solve the problem of insufficient compliance of traditional monoclonal antibodies. In the same year, Johnson & Johnson spent US$1.25 billion to acquire Yellow Jersey and obtained the bispecific antibody NM26 targeting atopic dermatitis.
Johnson & Johnson also introduced KP-723 from Japan's Kaken to develop STAT6 inhibitors, focusing on Th2-mediated inflammatory diseases, and competing with companies such as Sanofi and Kymera for the next generation of oral targeted therapies to further improve its coverage in this disease area.
From injectable formulations to oral peptides, from single targets to bispecific antibody platforms, Johnson & Johnson's autoimmune layout is not only an iteration of the product pipeline, but also a fusion of technological innovation and clinical needs, redefining the treatment paradigm of autoimmune diseases.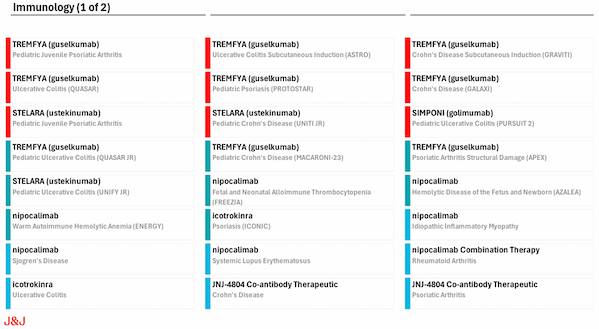 Some of Johnson & Johnson's autoimmune pipelines
Some of Johnson & Johnson's autoimmune pipelines
Image source: Johnson & Johnson 2024 financial report
4. Conclusion
Guselkumab was approved for the treatment of ulcerative colitis, becoming the first interleukin-23 inhibitor used in the treatment of ulcerative colitis in China. This is an important milestone in the history of UC treatment in China. It not only provides a new precision treatment option for moderate to severe patients, but also marks the deep integration of China's autoimmune disease treatment field with global innovation.
https://news.yaozh.com/archive/45432.html
By editorRead more on
- Rovaxitinib approved for marketing, filling the demand for myelofibrosis treatment March 2, 2026
- Warrant Pharmaceuticals’ active pharmaceutical ingredient receives Brazil’s first official GMP certification March 2, 2026
- Merck’s New Story March 2, 2026
- Rongchang Biotechnology has turned a profit! March 2, 2026
- Jiuyuan Gene’s “Simeglucopyranoside” for weight loss (Jikeqin®) has been submitted for market approval March 2, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



