JCI | Zhu Yingjie’s team from Shenzhen Advanced Institute reveals a new central target of GLP-1R agonist, a “weight loss drug”
September 5, 2024
Source: drugdu
 262
262
Obesity, as one of the top ten chronic diseases worldwide, has a research history of over a century in the field of weight loss drugs. After 2014, glucagon like peptide-1 (GLP-1) receptor agonists emerged as a new force, far surpassing other weight loss drugs in terms of efficacy and safety, and also driving the development and marketing of weight loss drugs.
GLP-1, as an intestinal insulinotropic hormone, is generated by the post-translational cleavage of pre glucagon encoded by the pre glucagon gene (GCG). It is mainly secreted by intestinal L cells and a portion of neurons in the solitary tract nucleus (NTS) of the brainstem. The action of GLP-1 is mediated by the GLP-1 receptor (GLP-1 R) and is widely distributed in the peripheral and central nervous systems. Liraglutide is a short acting GLP-1 receptor agonist that acts on the central nervous system to reduce appetite and slow gastric emptying. It is the first GLP-1 based anti obesity drug to enter the market. Although GLP-1R is widely expressed in the brain, the specific neural mechanisms of its agonist drugs in regulating food intake and weight are still not fully understood.
On September 3, Zhu Yingjie's team from the Institute of Brain Cognition and Brain Disease of the Chinese Academy of Sciences Shenzhen Institute of Advanced Technology/Shenzhen Hong Kong Institute of Brain Science Innovation published a research paper entitled "GLP-1R – positive neurons in the local segment medium the neural and weight powering effects of lithium in mice" on the Journal of Clinical Investigation. This study revealed the key role of GLP-1R positive neurons in the lateral septum (LS) of the brain in the anorexigenic and weight loss functions of liraglutide.

Article online screenshot
Previous studies on the mechanism of action of GLP-1 drugs have mostly focused on the hypothalamus (such as the arcuate nucleus, paraventricular nucleus, and medial nucleus) or the dorsal vagus nerve complex in the hindbrain. In the early stage, Zhu Yingjie's research group used cutting-edge technologies such as single-cell sequencing and optogenetics to systematically analyze the lateral septal nucleus (LS) brain region, and found that Nts positive neurons in this brain region regulate hedonic eating (Chen Z. et al., 2022 Molecular Psychiatry), and Esr1 positive neurons regulate methamphetamine reward effects (Chen G. et al., 2024 Neuron). During the research process, the research team noticed a large number of GLP-1R positive neurons in the LS region. Considering the important role of the LS brain region in regulating eating, researchers speculate whether the GLP-1R neurons of LS are involved in mediating the appetite suppressing and weight loss effects of liraglutide.
Through a series of experiments, researchers found that GLP-1R positive neurons (LSGLP-1R neurons) in the LS brain area were significantly activated after local or systemic administration of liraglutide. By knocking out GLP-1R in the LS brain region, the research team observed a significant reduction in the anorexia and weight loss effects of liraglutide, indicating that GLP-1R in the LS brain region is a new central target of liraglutide.
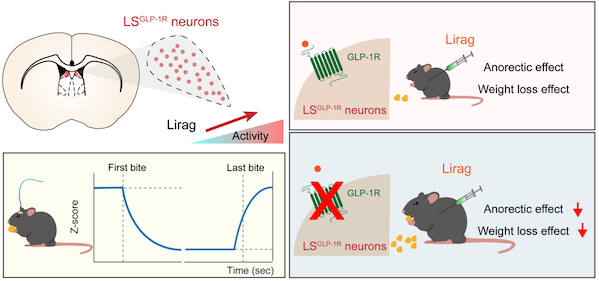
Research schematic diagram
Further research has found that the activity of LSGLP-1R neurons significantly decreases at the beginning of feeding and remains at a low level during the feeding process until it returns to baseline levels after feeding ends. Activating neuronal activity through optogenetics and chemogenetics methods can simulate the effects of liraglutide, significantly reducing food intake and weight loss. When the group of neurons is inactivated, the effect of liraglutide is significantly weakened. This study provides new insights and inspirations for understanding the neural mechanisms of eating behavior, developing new drugs for treating eating disorders and obesity, and studying the GLP-1R signaling pathway.
Associate researcher Chen Zijun and assistant researcher Deng Xiaofei from Zhu Yingjie's research group are the co first authors of the paper, and researcher Zhu Yingjie is the corresponding author of the paper.
By editorRead more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



