IASO Bio’s IND application for new autoimmune indication of Equecabtagene Autoleucel Injection approved
January 27, 2024
Source: drugdu
 330
330
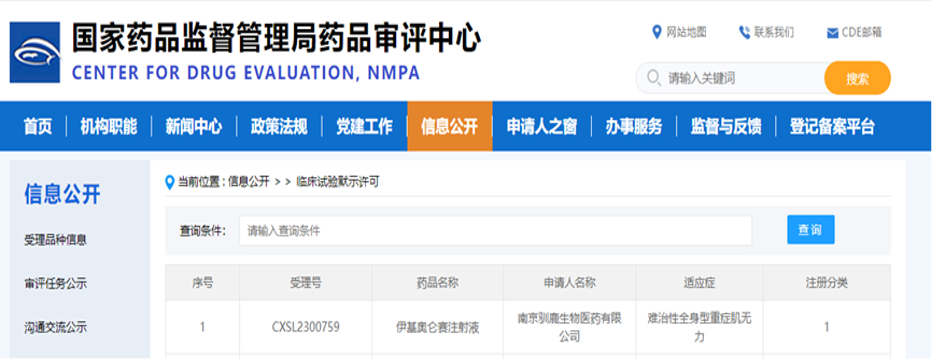 January 25, 2024, Nanjing, Shanghai, China, and San Jose, California, USA - IASO Bio, a biopharmaceutical company dedicated to the development, production and sales of innovative cell-based drugs, announced the National Medical Products Administration (NMPA) Review Center (CDE) has officially approved its fully human BCMA-targeted chimeric antigen receptor autologous T cell injection (Equecabtagene Autoleucel Injection, R&D code: CT103A) for the new expanded indication of refractory generalized myasthenia gravis (Myasthenia gravis, MG). ) clinical trial application (IND) (acceptance number: CXSL2300759).
January 25, 2024, Nanjing, Shanghai, China, and San Jose, California, USA - IASO Bio, a biopharmaceutical company dedicated to the development, production and sales of innovative cell-based drugs, announced the National Medical Products Administration (NMPA) Review Center (CDE) has officially approved its fully human BCMA-targeted chimeric antigen receptor autologous T cell injection (Equecabtagene Autoleucel Injection, R&D code: CT103A) for the new expanded indication of refractory generalized myasthenia gravis (Myasthenia gravis, MG). ) clinical trial application (IND) (acceptance number: CXSL2300759).
Equecabtagene Autoleucel Injection (trade name: Equecabtagene Autoleucel®) has been approved for marketing by the State Food and Drug Administration on June 30, 2023, for the treatment of relapsed and refractory multiple myeloma. The approval of this IND for myasthenia gravis further expands This is the second autoimmune indication approved for IND after Neuromyelitis Optica Spectrum Disease (NMOSD). IASO Bio is the first company in China to use CAR-T products for autoimmune indications, and is expected to change the treatment landscape of autoimmune diseases.
About myasthenia gravis
Myasthenia gravis (MG) is a disease of neuromuscular junction transmission disorder mediated by autoantibodies. The main clinical manifestation of MG is a decrease in muscle strength in local or systemic muscles, which can affect eye muscles, respiratory muscles, and limb muscles. and other important muscle groups, which have a huge negative impact on the patient's quality of life, and dysphagia or breathing caused by myasthenic crisis can be life-threatening. The main pathogenic antibodies of MG include AChR, MuSK and LRP4 antibodies. A very small number of patients have no detectable antibodies in their serum and are called antibody-negative MG1. MG can occur at any age and is slightly more common in women than in men2. According to relevant studies, the number of people with MG in China in 2022 will be about 9,600, and the number of patients with it will be about 282,0002,3. At present, most of the drugs used to treat MG in China are hormonal or non-hormonal immunosuppressives, but existing therapies can only provide relief and have shortcomings in disease control and long-term safety. 4
Ms. Zhang Jinhua, founder and CEO of IASO Bio, said: "For a long time, the treatment of patients with generalized myasthenia gravis has relied on expert experience and traditional treatment plans. Existing drug therapies are not effective for all patients. Clinically, 10 %-15% of MG patients have refractory disease. 4 The treatment of these refractory MG patients and patients who may potentially develop crises is an important clinical challenge, and safe and effective innovative treatments are urgently needed to solve these problems. In our IIT study, we are pleased to see that Equecabtagene Autoleucel Injection can arrest disease progression and show reversal of early signs of the disease, which is expected to change the MG treatment landscape. IASO Bio will initiate and complete clinical trials as soon as possible to provide support for myasthenia gravis in China Patients bring hope of cure.”
About IASO Bio
IASO Bio is a biopharmaceutical company dedicated to the development, production and sales of innovative cell-based drugs. The company takes the development of hematological tumor cell-based drugs and antibody drugs as the cornerstone of innovation and expands into autoimmune diseases. It has complete full-process capabilities from early discovery, clinical development, registration application to commercial production.
The company currently has more than 10 innovative drug varieties in different stages of research and development. Among them, Equecabtagene Autoleucel Injection (a fully human BCMA CAR-T product) has been approved for marketing by the National Medical Products Administration (NMPA) and has been approved by the US FDA for clinical registration. For the treatment of relapsed/refractory multiple myeloma.
With its strong management team, innovative product lines, in-house GMP production and strong clinical development capabilities, IASO Bio aims to provide transformative and curable innovative therapies and bring hope of cure to patients in China and even around the world. .
https://cn.iasobio.com/info.php?id=284
Read more on
- Marketing application for HSK39297 tablets, an innovative drug for paroxysmal nocturnal hemoglobinuria, has been accepted March 9, 2026
- Clinical trial application for olopatadine-mometasone nasal spray approved by NMPA March 9, 2026
- Superior to standard therapy! Johnson & Johnson’s blockbuster new drug combination therapy receives FDA approval March 9, 2026
- For the first time, a domestically produced ultra-long-acting TSLP bispecific antibody has been approved for clinical trials. March 9, 2026
- With Hengrui entering the fray, can Mingrui hold its ground? March 9, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



