$1.4 billion deal highlights the future of Alzheimer’s disease therapy
November 5, 2024
Source: drugdu
 518
518
The development of therapies for Alzheimer's disease has always caused many developers to feel exhausted, and now AbbVie has also increased its investment in neurodegenerative diseases.
AbbVie has agreed to spend $1.4 billion to acquire Aliada Therapeutics, whose main acquisition asset is Aliada's authorized Alzheimer's disease candidate drug ALIA-1758 from Johnson&Johnson. Its mechanism of action is still an anti beta amyloid protein (3pE-A β) antibody, but the unique feature of ALIA-1758 is that it can cross the blood-brain barrier, greatly improving the brain's absorption rate.
Aliada started a phase 1 trial among healthy volunteers in May, but AbbVie believes the timing is ripe for acquisition. Previously, AbbVie made the decision to abandon its internal Alzheimer's disease asset ABBV-916. This anti beta amyloid antibody has no significant difference in safety and efficacy compared to the already marketed monoclonal antibody therapy for Alzheimer's disease. Therefore, AbbVie decided to terminate the development of the project without any advantages. The acquired ALIA-1758 asset has the potential to become a best in class asset, with better ability to cross the blood-brain barrier than Lilly's Kisunla and Eisai/Bojian's Leqembi. ALIA-1758 was initially developed by Johnson&Johnson and designed using the proprietary Modular Delivery (MODEL) platform to cross the blood-brain barrier without triggering neurotoxicity. Rich transferrin receptor (TfR) or CD98 receptor (CD98) on the blood-brain barrier. MODEL utilizes this feature to fuse the anti TfR antibody module with Alzheimer's disease antibodies and bind to TfR receptors on the epithelial cells of the blood-brain barrier. This combination produces an endosomal compartment, which passes through the cytoplasm and enters the brain through endocytosis (Figure 1).
ALIA-1758 was initially developed by Johnson&Johnson and designed using the proprietary Modular Delivery (MODEL) platform to cross the blood-brain barrier without triggering neurotoxicity. Rich transferrin receptor (TfR) or CD98 receptor (CD98) on the blood-brain barrier. MODEL utilizes this feature to fuse the anti TfR antibody module with Alzheimer's disease antibodies and bind to TfR receptors on the epithelial cells of the blood-brain barrier. This combination produces an endosomal compartment, which passes through the cytoplasm and enters the brain through endocytosis (Figure 1).
ALIA-1758 has strong targeted binding properties and improved central nervous system delivery, which may enable effective clearance of brain beta amyloid protein at lower dose levels than other antibodies of the same class, and has the potential to manage Alzheimer's disease through subcutaneous low-dose regimens. In contrast, only 0.1% of the doses of donanemab and lecanemab, which have been marketed as Alzheimer's disease antibody drugs, can cross the blood-brain barrier.
The MODEL platform can not only carry antibodies, but also oligonucleotides, peptides, enzymes, and other proteins, crossing the blood-brain barrier by binding to TfR or CD98. The Fab fragment of ALIA-1758 has a high affinity for binding 3pE-A β, which may reduce the dosage level and target the plaque form of β - amyloid protein rather than the early stages of aggregation.
Optimized Fc fragments can prolong the half-life of antibodies and reduce effector function (the immune response triggered by the interaction between antibodies and the immune system). When transporting ALIA-1758, the MODEL platform used optimized Fc fragments to prevent antibody clearance through traditional immune activation pathways. On the contrary, the clearance process is achieved through a mechanism called non classical phagocytosis, which is accomplished through lysosomal degradation. Preclinical data shows that this unique clearance pathway can reduce cytokine release and other inflammatory markers. The potential for low-dose and subcutaneous injection, combined with this differentiated inflammatory response feature, may give ALIA-1758 unique efficacy and safety advantages over donanemab and lecanemab. The anti TfR delivery module is crucial for crossing the blood-brain barrier, delivering 3pE-A β antibodies to the brain through binding with TfR. The anti TfR delivery module can exist in various forms, such as scFv (single chain variable region fragment, Figure 2).
The anti TfR delivery module is crucial for crossing the blood-brain barrier, delivering 3pE-A β antibodies to the brain through binding with TfR. The anti TfR delivery module can exist in various forms, such as scFv (single chain variable region fragment, Figure 2).
Coincidentally, Roche has also chosen Brainshuttle, a technology platform that supports crossing the blood-brain barrier, in the development of Alzheimer's disease. This is a strategic choice made against the backdrop of Roche terminating multiple Alzheimer's disease pipeline projects.
Roche recently announced the termination of its collaboration with UCB to develop bepranemab, a candidate drug for Alzheimer's disease. This is the third time Roche has abandoned its Alzheimer's disease project this year. As early as January, Roche returned two jointly developed Alzheimer's disease assets to AC Immune. After terminating the bepranemab project, Roche shifted the focus of Alzheimer's disease therapy development entirely to their own pipeline product, trontinemab (RG6102).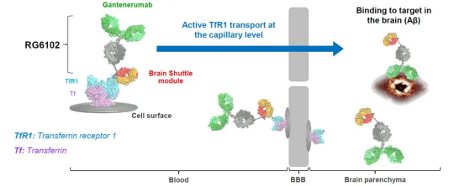 Trontinemab is designed and developed using Roche's Brainshuttle technology on the basis of the original anti amyloid monoclonal antibody gantenerumab, targeting and inhibiting β - amyloid protein. Similar to the MODEL acquired by AbbVie, Brainshuttle also utilizes TfR to cross the blood-brain barrier. Trontinemab developed on Brainshuttle technology is a 2+1 bispecific monomer that can bind bivalently to A β protein and monovalent to human TfR1 (Figure 3). The TfR1 binding domain of Trontinemab can bind to TfR1 receptors on the blood-brain barrier without disrupting the interaction between endogenous ligands and TfR1 receptors.
Trontinemab is designed and developed using Roche's Brainshuttle technology on the basis of the original anti amyloid monoclonal antibody gantenerumab, targeting and inhibiting β - amyloid protein. Similar to the MODEL acquired by AbbVie, Brainshuttle also utilizes TfR to cross the blood-brain barrier. Trontinemab developed on Brainshuttle technology is a 2+1 bispecific monomer that can bind bivalently to A β protein and monovalent to human TfR1 (Figure 3). The TfR1 binding domain of Trontinemab can bind to TfR1 receptors on the blood-brain barrier without disrupting the interaction between endogenous ligands and TfR1 receptors.
Recently, Eli Lilly has also obtained permission to use the Qinotto platform QinoTrans. QinoTrans technology also utilizes engineered proteins to bind to receptors on the blood-brain barrier, carrying therapeutic agents across the blood-brain barrier.
Earlier this year, Eisai also reached an agreement with Swedish biotechnology company BioArctic to develop Alzheimer's disease candidate drugs using the latter's technology platform BioArctic BrainTransporter technology. The core technology of BioArctic BrainTransporter is also to carry therapeutic agents across the blood-brain barrier.
The technology of crossing the blood-brain barrier plays a crucial role in the future development of therapies for Alzheimer's disease and other central nervous system diseases. The blood-brain barrier is a natural protective barrier of the brain that effectively blocks the entry of most large molecules, ensuring that the central nervous system is protected from external substances. But this characteristic also makes it extremely difficult to develop therapies for brain diseases, especially when treating diseases such as Alzheimer's disease, where the blood-brain barrier has become the main barrier to therapeutic drugs.
With the deepening of research on Alzheimer's disease, people are increasingly realizing that only by successfully breaking through the blood-brain barrier can drugs reach brain lesions and directly act on the key pathological mechanisms of Alzheimer's disease. However, due to the fact that most existing drugs cannot cross the blood-brain barrier, their effectiveness in actual treatment is greatly limited. Therefore, drugs with the ability to cross the blood-brain barrier can not only enhance the targeting of treatment, but also greatly improve patients' response to drugs, fundamentally changing the therapeutic effect.
A drug technology platform with the ability to cross the blood-brain barrier will become the core of Alzheimer's disease therapy development. The application of such technology platforms is not limited to Alzheimer's disease, but can also be extended to other neurodegenerative diseases such as Parkinson's disease, Huntington's disease, etc. This will greatly expand the development space of drugs for central nervous system diseases and provide new treatment methods for various refractory diseases.
By editorRead more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



