FDA Approves Two of Scientia Vascular’s Neurovascular Catheters
May 9, 2024
Source: drugdu
 332
332
Don Tracy, Associate Editor
The catheters approved by the FDA include the Plato 17, a DMSO-compatible microcatheter, and the Socrates 38, an aspiration catheter specifically designed for treating ischemic strokes.
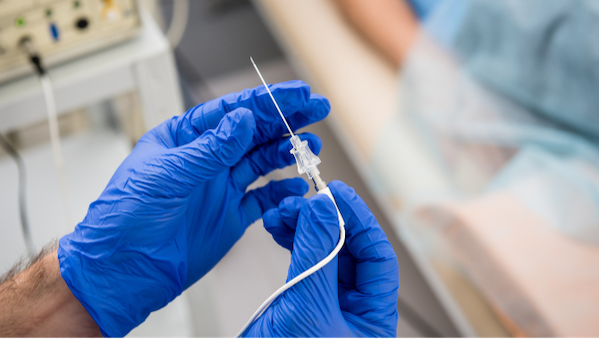 Scientia Vascular announced that the FDA has approved two of its neurovascular catheters. According to the company, the Plato 17 microcatheter offers physicians the ability to control and stabilize neurovascular applications and is also DMSO compatible. The Socrates 38, which is currently in a limited market release, is an aspiration catheter for treating ischemic strokes. Scientia stated that the approvals mark a significant advancement in its medical technology portfolio.1
Scientia Vascular announced that the FDA has approved two of its neurovascular catheters. According to the company, the Plato 17 microcatheter offers physicians the ability to control and stabilize neurovascular applications and is also DMSO compatible. The Socrates 38, which is currently in a limited market release, is an aspiration catheter for treating ischemic strokes. Scientia stated that the approvals mark a significant advancement in its medical technology portfolio.1
"Our FDA clearance is a significant milestone for Scientia Vascular. By applying proven microfabrication technology to catheters and designing our access and treatment devices for efficiency when used together, we're providing physicians the next generation of neurovascular access tools." said John Lippert, CEO, Scientia Vascular, in a press release. "I am immensely grateful to our dedicated teams whose tireless efforts have made this FDA clearance milestone possible. Their commitment to excellence and innovation continues to drive our patient mission forward."
In February, Scientia Vascular announced that Paul Fischer would be taking over as its chief commercial officer during a time the company considered to be highly successful.2
"We've seen what microfabrication can do in products like our Aristotle 24 and Colossus wires in changing the standard of care for patient treatment in stroke, so it's rewarding to see the excitement and buzz surrounding microfabricated technology in catheters with our physician community," said Fischer, in the press release. "We are eager to see how these advanced technologies will enhance patient care and offer new possibilities for physicians treating a variety of neurovascular disease states."
According to Precedence Research, the market for neurovascular catheters is expected to reach approximately $11 billion by 2033, previously evaluated at over $5 billion last year. The company credits this to the increasing occurrences of neurological disorders, which in turn increases the demand for medical devices, such as microcatheters. Additionally, a study by the company suggests that the growing market is also a result of catheters being utilized for neurovascular conditions, such as cerebral aneurysms and ischemic stroke.
According to researchers, the frequency of neurovascular diseases in the United States is a major player when it comes to neurovascular catheters, which work to guide interventional devices for neurovascular therapy, addressing lesions or procedural sites through percutaneous intravascular procedures within the neurovasculature.3
“The neurovascular catheters market is experiencing rapid growth due to their pivotal role in treating conditions affecting blood vessels within the brain through minimally invasive procedures,” reports Precedence Research. “These catheters are inserted via femoral or brachial arteries and manually navigated to deliver treatment to the brain, eliminating the need for invasive skull and brain tissue incisions. However, challenges arise from the reduction of blood vessel diameter and increased tortuosity in deep brain vessels, limiting procedure effectiveness. Despite these challenges, neurovascular catheters have revolutionized patient treatment worldwide, advancing healthcare in this critical field.”
Read more on
- //news.yaozh.com/archive/47318.html PD-1 sales surge March 4, 2026
- A major breakthrough! Roche’s oral BTK inhibitor achieves its third Phase III clinical trial victory, a game-changer in the multi-billion dollar MS (manufactured pharmaceuticals) market. March 4, 2026
- GB19 Injection Approved for Clinical Trials of Cutaneous Lupus Erythematosus March 4, 2026
- First patient dosing completed for KRAS G12D inhibitor ABSK141 On the morning of March 4th, Heyu -B (02256) announced that its subsidiary, Shanghai Heyu Biopharmaceutical Technology Co., Ltd., has successfully completed the first patient dosing in a Phase I/II clinical trial of its oral, highly effective, and selective small molecule KRAS G12D inhibitor, ABSK141, for patients with advanced solid tumors harboring KRAS G12D mutations. This progress marks a significant milestone for Heyu Pharmaceuticals in the field of KRAS-targeted therapy, and is expected to provide a transformative potential treatment option for patients with KRAS G12D-mutated solid tumors. According to the announcement, KRAS is one of the most common oncogenes in human cancers, with G12D being the major mutation subtype, widely found in various solid tumors such as pancreatic ductal adenocarcinoma, colorectal cancer, and non-small cell lung cancer. Because G12D mutations lead to specific conformational changes in the KRAS protein, it has long been considered an “untreatable” target, and currently, no targeted therapies against KRAS G12D mutations have been approved globally. The announcement indicates that the first patient dose was administered in an open-label Phase I/II clinical trial, which is expected to receive approval for a new drug clinical trial from the U.S. Food and Drug Administration and the China National Medical Products Administration in December 2025. ABSK141 is a novel oral small-molecule KRAS G12D inhibitor independently designed and developed by Heyu Pharmaceuticals. It selectively binds to the KRAS G12D mutant protein, blocking downstream signaling pathways and inhibiting tumor cell proliferation. Preclinical studies have shown that ABSK141 exhibits potentially best-in-class oral bioavailability in various animal models and demonstrates significant antitumor activity in multiple human tumor cell line xenograft models, including pancreatic ductal adenocarcinoma and colorectal cancer. The announcement stated that Heyu Pharmaceuticals, established in April 2016, is a biopharmaceutical company focused on the field of oncology, headquartered in Shanghai, and dedicated to the discovery and development of innovative drugs.The company aims to address unmet medical needs in China and globally. Its founders and management team are all seasoned drug development experts with extensive R&D and management experience from leading multinational pharmaceutical companies. Since its inception, Heyu Pharmaceuticals has built a rich pipeline of innovative products, focusing on precision oncology and immuno-oncology field. 网址:https March 4, 2026
- China Sino Biopharmaceutical Signs Exclusive Licensing Agreement with Sanofi for Rofalcitinib March 4, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



