FDA approves Sun Pharma’s JAK inhibitor for alopecia
July 29, 2024
Source: drugdu
 433
433
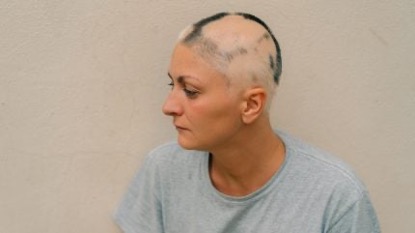 Sun Pharmaceutical Industries has secured the US Food and Drug Administration (FDA) approval for its oral Janus kinase (JAK) Inhibitor Leqselvi (deuruxolitinib) to treat severe alopecia areata.
Sun Pharmaceutical Industries has secured the US Food and Drug Administration (FDA) approval for its oral Janus kinase (JAK) Inhibitor Leqselvi (deuruxolitinib) to treat severe alopecia areata.
Originally developed by Concert Pharmaceuticals, Sun Pharma inherited the asset when it purchased Concert Pharmaceuticals in January 2023 for $576m.
Alopecia areata is an autoimmune condition where the immune system attacks hair follicles, leading to sudden, patchy hair loss. JAK inhibitors such as Leqselvi block the activity of Janus kinase enzymes, which are involved in the signalling pathways that drive inflammation and immune responses.
The FDA approval is based on data gathered from two Phase III studies—THRIVE-AA1 and THRIVE-AA2 (NCT04518995 and NCT04797650)—where Leqselvi restored scalp hair coverage by at least 80% in more than 30% of patients after 24 weeks. The 1,220 patients enrolled across the two studies had alopecia areata with at least 50% scalp hair loss as measured by Severity of Alopecia Tool (SALT) scale for more than six months.
JAK inhibitors have gained significant attention in the immunology space. Pharma giants such as Pfizer and AbbVie stacked up on approvals in indications such as atopic dermatitis, ulcerative colitis, and rheumatoid arthritis with their blockbuster JAK inhibitors Xeljanz (tofacitinib citrate) and Rinvoq (upadacitinib), respectively.
Alopecia areata, which was highlighted as an unmet need by professionals in the space, is now another area of expansion. Eli Lilly’s Olumiant (baricitinib) bagged an FDA approval in this space in 2022, making it the first approved JAK inhibitor to treat the condition. Before this, patients would seek local corticosteroid injections as off label treatments. Olumiant pulled in $922.6m in sales for Lilly in 2023, as per the company’s financials.
In September 2021, the FDA said it will require safety warnings for JAK inhibitors after a study in rheumatoid arthritis patients showed an increased risk of cardiovascular events. Similar to most other JAK inhibitors, Leqselvi comes with a boxed warning for serious infections, mortality, malignancies, major adverse cardiovascular events, and thrombosis.
In the announcement accompanying the approval, Sun Pharma’s North America CEO Abhay Gandhi said: “Leqselvi offers a new and effective solution that will significantly enhance options for long-suffering patients battling severe alopecia areata and their physicians. Our fast-growing dermatology business is excited to add this novel treatment to its portfolio.”
According to GlobalData’s Pharma Intelligence Center, Leqselvi is forecast to generate $253m in 2030. GlobalData is the parent company of Pharmaceutical Technology.
https://www.pharmaceutical-technology.com/news/fda-approves-sun-pharmas-jak-inhibitor-for-alopecia/?cf-view
Read more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



