FDA Approves Stelara Biosimilar Selarsdi to Treat Psoriasis, Psoriatic Arthritis
April 19, 2024
Source: drugdu
 365
365
Davy James
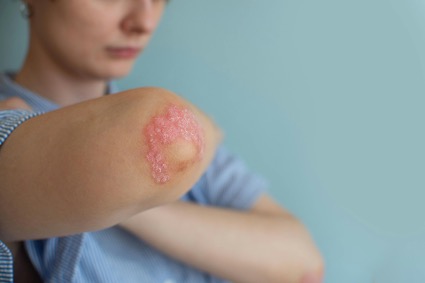 Alvotech’s and Teva's Selarsdi (ustekinumab-aekn), the second FDA-approved biosimilar to Stelara, is indicated to treat patients aged 6 years and above with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and for patients aged 6 years and above with active psoriatic arthritis.
Alvotech’s and Teva's Selarsdi (ustekinumab-aekn), the second FDA-approved biosimilar to Stelara, is indicated to treat patients aged 6 years and above with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and for patients aged 6 years and above with active psoriatic arthritis.
Image credit: Maria | stock.adobe.com
Alvotech’s and Teva Pharmaceuticals' Selarsdi (ustekinumab-aekn) has been approved by the FDA as a biosimilar to Stelara (ustekinumab), which represents the second approval for a Stelara biosimilar.1 Selarsdi is indicated to treat patients aged 6 years and above with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy; and for patients aged 6 years and above with active psoriatic arthritis.
“The approval of Selarsdi—which is our second biosimilar approval this year—underscores Teva’s commitment to expanding the availability, access and uptake of this important treatment option to patients in the US,” Thomas Rainey, Teva senior vice president of US Market Access, said in a press release. “The biosimilars market is growing, both globally and in the US, and biosimilars are a key component of delivering on Teva’s Pivot to Growth strategy. The partnership model that we’ve established enables us to leverage our commercial presence and experiences globally as we move to bring additional biosimilars to market.”1
Stelara, a humanized immunoglobulin G1k (IgG1k) monoclonal antibody, is indicated to treat conditions such as moderate to severe plaque psoriasis, psoriatic arthritis, Crohn disease, and ulcerative colitis.
In November 2023, Wezlana (ustekinumab-auub; Amgen) became the first FDA-approved biosimilar to Stelara for the treatment of inflammatory diseases. The FDA approved Wezlana as an interchangeable biosimilar to Stelara after it showed no clinically meaningful differences in safety and efficacy for the reference product’s indications.2
The FDA based the approval of Selarsdi on a totality of evidence from the biosimilar’s clinical development program, which included data from the randomized, double blind, multicenter, 52-week Study AVT04-GL-301 (NCT04930042). Selarsdi achieved the trial’s primary endpoint of percent improvement in Psoriasis Area and Severity Index score from baseline to week 12 in 581 patients with moderate to severe chronic plaque-type psoriasis.
Also included in the clinical development program was the randomized, double-blind, single-dose, parallel-group, three-arm Phase I Study AVT04-GL-101 (NCT04744363). The trial compared the pharmacokinetic, safety, tolerability, and immunogenicity profiles of a single 45 mg/0.5 mL subcutaneous injection of Selarsdi vs. the European- and US-approved versions of Stelara in 294 patients.
As part of the strategic partnership agreement reached between Teva and Alvotech, Teva will be responsible for the exclusive commercialization of the biosimilar in the United States. In June 2023, Alvotech and Teva reached a settlement and license agreement with Johnson & Johnson, the manufacturer of Stelara, which gave the biosimilar a license entry date in the United States no later than February 21, 2025.3
“We are delighted to announce our second biosimilar approval in the US, which is the thirty-eighth approved market for our biosimilar to Stelara globally,” said Robert Wessman, chairman and CEO of Alvotech, in the press release. “Bringing Selarsdi to market in the US early next year presents a significant opportunity to improve patient access to a vital biologic in inflammatory disease and contribute to the reduction of inflationary pressure in healthcare costs. The development of Selarsdi leveraged our purpose-built end-to-end development and manufacturing platform for biosimilars. Being able to develop the biosimilar in the same cell type and continuous perfusion process as was used for the reference product, facilitated the development program’s success.”
Read more on
- Gusekirumab Injection Accepted by CDE, Multiple Pipelines Advancing Simultaneously March 4, 2026
- Yifan Pharmaceutical’s teriparatide injection has been accepted by the CDE (Center for Drug Evaluation), adding a new domestic player to the osteoporosis treatment field March 4, 2026
- //news.yaozh.com/archive/47318.html PD-1 sales surge March 4, 2026
- A major breakthrough! Roche’s oral BTK inhibitor achieves its third Phase III clinical trial victory, a game-changer in the multi-billion dollar MS (manufactured pharmaceuticals) market. March 4, 2026
- GB19 Injection Approved for Clinical Trials of Cutaneous Lupus Erythematosus March 4, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



