EMBO J | Jie Yunli team reveals heterogeneity mechanism of glial cells
September 24, 2024
Source: drugdu
 343
343
BioArt
In the brains of mammals, in addition to a large number of neurons, there are also a large number of glial cells. These glial cells are widely involved in various aspects of advanced brain functions. Among them, astrocytes, as one of the most abundant glial cells in the mammalian brain, play a crucial role in the functioning of the brain, including participating in synaptic pruning, formation of the blood-brain barrier, and regulation of brain homeostasis balance. Moreover, an increasing number of studies indicate that astrocytes are directly involved in the processing of neural circuit information, and their developmental disorders may trigger various brain diseases. It is worth noting that the morphology and transcriptome characteristics of astrocytes exhibit high heterogeneity in different brain regions and even within the same brain region. During the development of the brain, the origin of astrocytes, namely the location of neural stem cells, determines the distribution of astrocytes in the brain, which is considered one of the reasons for the heterogeneity of astrocytes. However, the mechanism by which the distribution of astrocytes in the brain is regulated during brain development is currently unclear.
Recently, the team led by Jie Yunli from the Institute of Neuroscience at Fudan University published an article titled 'Astrocyte allocation during brain development is controlled by Tcf4 mediated fat restriction' in The EMBO journal. This study reveals that transcription factor Tcf4 restricts the fate of oligodendrocyte precursor cells derived from ventral brain neural stem cells, inhibits the fate of astrocytes, and precisely regulates the localization of astrocytes in the dorsal neocortex. Previous research in the laboratory has shown that the precise localization of embryonic neural precursor cells in the cortex plays an important role in cortical development, highlighting the importance of spatial location of embryonic neural precursor cells in brain development. Mutations in transcription factor TCF4 can lead to Pitt Hopkins syndrome on the autism spectrum, which is often accompanied by symptoms such as intellectual disability, developmental disorders, and delayed language development. The previous research by Jie Yunli's team found that TCF4 is crucial for maintaining the localization of excitatory neurons in the cortex. TCF4 deficient mice exhibit clustered distribution of cortical neurons [2], which is consistent with the early detection of neuronal clustering in the cortex of some children with autism [3]. Meanwhile, TCF4 also participates in the migration of hippocampal neurons [4]. Based on the previous research findings that TCF4 regulates the number of oligodendrocytes in the olfactory bulb, the team used a mouse model and combined it with omics techniques such as Cut Tag, single-cell sequencing, and ATAC seq to find that TCF4 can inhibit the expression of astrocyte genes in oligodendrocytes, limiting the fate of glial cells derived from the ventral hemisphere to oligodendrocytes and avoiding their distribution in the dorsal neocortex. By combining tissue transparency technology and astrocyte calcium imaging technology, it was found that the characteristics of astrocytes originating from the ventral telencephalon are more similar to those produced by dorsal neural stem cells, but differ from homologous ventral astrocytes. These results suggest that astrocytes derived from the ventral telencephalon are influenced by the dorsal cortical environment, suggesting that astrocytes have a certain degree of plasticity.
Previous research in the laboratory has shown that the precise localization of embryonic neural precursor cells in the cortex plays an important role in cortical development, highlighting the importance of spatial location of embryonic neural precursor cells in brain development. Mutations in transcription factor TCF4 can lead to Pitt Hopkins syndrome on the autism spectrum, which is often accompanied by symptoms such as intellectual disability, developmental disorders, and delayed language development. The previous research by Jie Yunli's team found that TCF4 is crucial for maintaining the localization of excitatory neurons in the cortex. TCF4 deficient mice exhibit clustered distribution of cortical neurons [2], which is consistent with the early detection of neuronal clustering in the cortex of some children with autism [3]. Meanwhile, TCF4 also participates in the migration of hippocampal neurons [4]. Based on the previous research findings that TCF4 regulates the number of oligodendrocytes in the olfactory bulb, the team used a mouse model and combined it with omics techniques such as Cut Tag, single-cell sequencing, and ATAC seq to find that TCF4 can inhibit the expression of astrocyte genes in oligodendrocytes, limiting the fate of glial cells derived from the ventral hemisphere to oligodendrocytes and avoiding their distribution in the dorsal neocortex. By combining tissue transparency technology and astrocyte calcium imaging technology, it was found that the characteristics of astrocytes originating from the ventral telencephalon are more similar to those produced by dorsal neural stem cells, but differ from homologous ventral astrocytes. These results suggest that astrocytes derived from the ventral telencephalon are influenced by the dorsal cortical environment, suggesting that astrocytes have a certain degree of plasticity.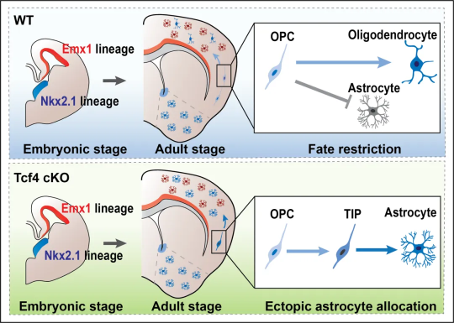 Pitt Hopkins syndrome is a rare autism spectrum disorder caused by mutations in the transcription factor TCF4. Although clinical studies have shown that it is caused by TCF4 mutations, the pathogenic mechanism is still unclear. Previous studies, including those by Jie Yunli's team, have mostly focused on the role of TCF4 in neurons. And this work first discovered the regulatory effect of TCF4 on glial cell development, providing new ideas for understanding the pathogenesis of Pitt Hopkins syndrome.
Pitt Hopkins syndrome is a rare autism spectrum disorder caused by mutations in the transcription factor TCF4. Although clinical studies have shown that it is caused by TCF4 mutations, the pathogenic mechanism is still unclear. Previous studies, including those by Jie Yunli's team, have mostly focused on the role of TCF4 in neurons. And this work first discovered the regulatory effect of TCF4 on glial cell development, providing new ideas for understanding the pathogenesis of Pitt Hopkins syndrome.
The corresponding author of this paper is Jie Yunli's Institute of Neuroscience at Fudan University, and Zhang Yandong, Li Dan, and Cai Yuqun from Jie Yunli's research group are co first authors. Other members and collaborators of the laboratory also made significant contributions during the course of the project.
By editorRead more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



