Edwards receives FDA approval for first transcatheter tricuspid valve replacement treatment
February 4, 2024
Source: drugdu
 371
371
Dive Brief
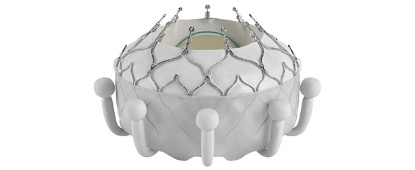
Edwards Lifesciences received approval from the Food and Drug Administration for its Evoque tricuspid valve replacement system, making it the first company to bring a transcatheter treatment for tricuspid regurgitation to the U.S.
Tricuspid regurgitation is a condition where the valve between the two right heart chambers doesn’t close properly, allowing blood to leak backward. Edwards’ Evoque device is inserted through a minimally invasive surgery and is designed to replace the tricuspid valve.
Competitor Abbott has submitted a device for FDA approval called TriClip, which clips together a portion of the leaflets of the tricuspid valve to prevent blood from leaking back. The FDA plans to hold an advisory panel on Feb. 13 to discuss the device.
Dive Insight
The approval for Evoque is a “meaningful milestone for [Edwards] as well as for the industry,” Shagun Singh, an RBC Capital Markets analyst, wrote in a research note.
The FDA’s approval came earlier than expected, with Edwards forecasting a decision by mid-2024, Singh wrote. It was based on the results of Edwards’ Triscend II pivotal trial, which found that 98.8% of patients achieved moderate or lower tricuspid regurgitation after six months and had an adverse event rate of 27.4% after 30 days.
Edwards, which announced the FDA approval Friday, also received a CE Mark for the device in October.
Singh expects adoption of the device to be more gradual than with transcatheter aortic valve replacement, given different disease etiologies and patient anatomies and the lack of a parallel surgery pool.
“Our checks also suggest that it is early for the field as more clinical evidence is established, and as doctors assess which patients are best suited for such therapies. That said, this is a highly underserved population as most tricuspid patients have limited to no treatment options today,” Singh wrote.
Currently, tricuspid valve repair is usually done with open-heart surgery or managed using medications to control symptoms. Edwards’ device is indicated for patients with symptomatic severe tricuspid regurgitation despite treatment with medications.
Other minimally invasive treatments are also on the horizon. BTIG analyst Marie Thibault expects Abbott’s TriClip G4 will be approved given positive safety and efficacy data from its Triluminate pivotal study.
“We are not sure why an FDA panel is being convened for TriClip, but Evoque was approved without a panel,” Thibault wrote in a research note.
Edwards’ Evoque system is part of its transcatheter mitral and tricuspid therapies segment, which brought in $52 million in sales in the third quarter.
Source:
https://www.medtechdive.com/news/edwards-fda-approval-evoque-tricuspid-valve/706429/
Read more on
- Gusekirumab Injection Accepted by CDE, Multiple Pipelines Advancing Simultaneously March 4, 2026
- Yifan Pharmaceutical’s teriparatide injection has been accepted by the CDE (Center for Drug Evaluation), adding a new domestic player to the osteoporosis treatment field March 4, 2026
- //news.yaozh.com/archive/47318.html PD-1 sales surge March 4, 2026
- A major breakthrough! Roche’s oral BTK inhibitor achieves its third Phase III clinical trial victory, a game-changer in the multi-billion dollar MS (manufactured pharmaceuticals) market. March 4, 2026
- GB19 Injection Approved for Clinical Trials of Cutaneous Lupus Erythematosus March 4, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



