Domestically developed blockbuster ADC approved for new indication
February 10, 2026
Source: drugdu
 62
62
 On February 6, the NMPA website showed that Kelun Biotech's TROP2 ADC, sasugamucil ( sac-TMT), has been approved for a new indication: the treatment of adult patients with unresectable locally advanced or metastatic hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer (BC) who have previously received endocrine therapy and other systemic therapies at an advanced or metastatic stage . This indication had previously been included in the priority review and approval process by the CDE (Center for Drug Evaluation).
On February 6, the NMPA website showed that Kelun Biotech's TROP2 ADC, sasugamucil ( sac-TMT), has been approved for a new indication: the treatment of adult patients with unresectable locally advanced or metastatic hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer (BC) who have previously received endocrine therapy and other systemic therapies at an advanced or metastatic stage . This indication had previously been included in the priority review and approval process by the CDE (Center for Drug Evaluation).
Screenshot source: NMPA official website
Sacubituzumab is an ADC drug targeting TROP2 developed by Kelun Biotech. It is composed of a humanized monoclonal antibody, Sacituzumab, an enzymatically cleavable linker, and a novel topoisomerase I inhibitor, T030, with an average drug-to-antibody ratio (DAR) as high as 7.4.
In May 2022, Kelun Biotech and Merck signed a collaboration agreement, granting the latter exclusive rights to develop, use, manufacture, and commercialize luconsulamutuzumab in all regions outside of Greater China . In November 2025, Merck announced that Blackstone would pay $700 million ( non-refundable, subject to termination clauses in the agreement ) to cover a portion of the research and development costs of luconsulamutuzumab in 2026.
To date, Merck has conducted 16 global Phase III clinical trials of luconsultuzumab in multiple areas, including lung cancer, breast cancer, gastric cancer, and gynecological tumors .
Regarding regulatory review, luconsulotocillin has previously been approved for three indications in China , and this is the fourth.
In November 2024, it was approved for use in patients with locally advanced or metastatic triple-negative breast cancer (TNBC) who have previously received at least two systemic therapies (at least one of which was for an advanced or metastatic stage) .
In March 2025, it was approved for the treatment of adult patients with EGFR gene mutation-positive locally advanced or metastatic non-squamous NSCLC who have progressed after EGFR-TKI and platinum-based chemotherapy .
In October 2025, it was indicated for the treatment of adult patients with EGFR-mutant locally advanced or metastatic NSCLC who had progressed after treatment with an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) .
This approval for a new indication is based on positive results from the OptiTROP-Breast02 registration phase III study . This was a randomized, open-label, multicenter phase III clinical trial evaluating the efficacy and safety of lucacanthosum monotherapy (5 mg/kg, Q2W) versus investigator-selected chemotherapy regimens for the treatment of patients with locally advanced or metastatic HR+/HER2- (IHC 0, IHC 1+, or IHC 2+/ISH-) BC.The results of the OptiTROP-Breast02 study were officially released at the ESMO Congress in 2025. In this study, a total of 399 HR+/HER2- BC patients who had previously received CDK4/6 inhibitor therapy and had progressed after receiving at least one chemotherapy regimen in the advanced or metastatic stage were randomly assigned (1:1) to receive either rucalcanesartuzumab or investigator-selective chemotherapy (ICC) .Data up to January 22, 2025. Results showed that the median PFS in the luconsastuzumab group was significantly longer than that in the ICC group (8.3 months vs. 4.1 months) , and clinical benefit was observed in patients with different HER2 expression levels (HR=0.39 for patients without HER2 expression; HR=0.31 for patients with low HER2 expression) . 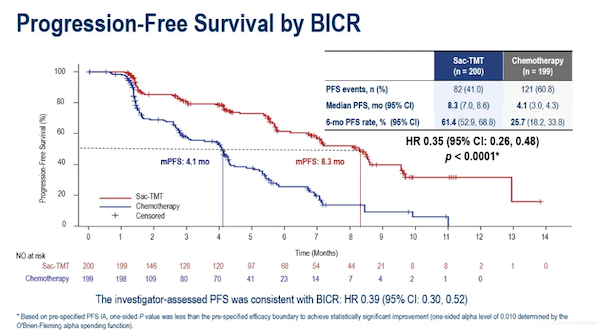 Compared with chemotherapy, luconsautuzumab showed a longer duration of response (DoR) and its ORR was also better than that of the ICC group ( 41.5% vs 24.1% ) ; in terms of OS, the luconsautuzumab group showed a more favorable trend compared with the ICC group (HR=0.33) .
Compared with chemotherapy, luconsautuzumab showed a longer duration of response (DoR) and its ORR was also better than that of the ICC group ( 41.5% vs 24.1% ) ; in terms of OS, the luconsautuzumab group showed a more favorable trend compared with the ICC group (HR=0.33) .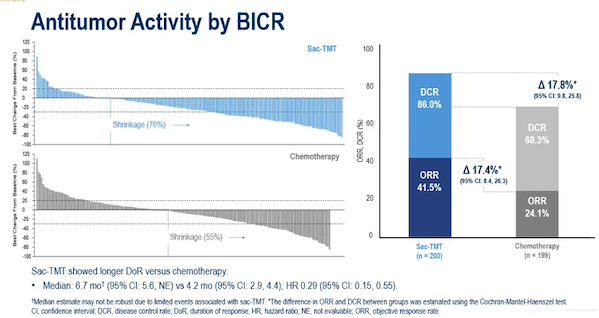 Regarding safety, 62.0% of patients in the luconsatuzumab group and 64.8% of patients in the ICC group experienced grade 3 or higher treatment-related adverse events (TRAEs) . 0% of patients in the luconsatuzumab group and 0.5% of patients in the ICC group discontinued treatment due to TRAEs; 1.5% of patients in the luconsatuzumab group and 1.0% of patients in the ICC group experienced pneumonia cases (all grade 1-2).
Regarding safety, 62.0% of patients in the luconsatuzumab group and 64.8% of patients in the ICC group experienced grade 3 or higher treatment-related adverse events (TRAEs) . 0% of patients in the luconsatuzumab group and 0.5% of patients in the ICC group discontinued treatment due to TRAEs; 1.5% of patients in the luconsatuzumab group and 1.0% of patients in the ICC group experienced pneumonia cases (all grade 1-2).
Currently, three TROP2 ADCs have been approved globally: Luconsulotocillin (Colomb Biotech), Gosatuzumab (Gilead), and Dedabrotuzumab (Daiichi Sankyo/AstraZeneca) . Domestically, in addition to the approved Luconsulotocillin, three other ADCs have entered Phase III clinical trials, from Fudan Zhangjiang, Hengrui, and Shijian Bio/Lianning Bio , respectively .
https://bydrug.pharmcube.com/news/detail/5bb801884b8a78acdf5e240572604e27
By editorRead more on
- Gusekirumab Injection Accepted by CDE, Multiple Pipelines Advancing Simultaneously March 4, 2026
- Yifan Pharmaceutical’s teriparatide injection has been accepted by the CDE (Center for Drug Evaluation), adding a new domestic player to the osteoporosis treatment field March 4, 2026
- //news.yaozh.com/archive/47318.html PD-1 sales surge March 4, 2026
- A major breakthrough! Roche’s oral BTK inhibitor achieves its third Phase III clinical trial victory, a game-changer in the multi-billion dollar MS (manufactured pharmaceuticals) market. March 4, 2026
- GB19 Injection Approved for Clinical Trials of Cutaneous Lupus Erythematosus March 4, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



