Domestic bispecific antibodies win another victory
November 11, 2024
Source: drugdu
 229
229
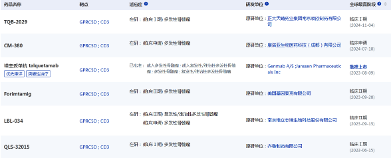 Recently, Wellizbo announced that its independently developed and globally intellectual property-owned Class 1 new drug LBL-034 has been granted orphan drug designation by the U.S. Food and Drug Administration (FDA) for the treatment of multiple myeloma (MM).
Recently, Wellizbo announced that its independently developed and globally intellectual property-owned Class 1 new drug LBL-034 has been granted orphan drug designation by the U.S. Food and Drug Administration (FDA) for the treatment of multiple myeloma (MM).
LBL-034 is a new generation of humanized bispecific T cell engaging antibodies targeting GPRC5D and CD3 developed using Wellizbo's independently developed and intellectual property-owned CD3 bispecific antibody technology platform "LeadsBodyTM". It is the third CD3 T cell connector targeting GPRC5D to enter the clinical stage in the world.
Multiple myeloma (MM) is a malignant plasma cell disease caused by abnormal proliferation of clonal plasma cells, accounting for about 10% of hematological malignancies and about 1% of all tumor diseases. MM is still an incurable malignancy. With multiple lines of medication, the interval between relapses will become shorter and shorter, and eventually evolve into RRMM, which seriously threatens the life and health of patients. It is urgent to develop new and more effective treatment options.
According to a press release from Wellizbo, LBL-034 selectively binds to T cells only in the presence of GPRC5D+ cells, thereby conditionally activating T cells in the tumor microenvironment where GPRC5D is expressed. In cells with high, medium and low GPRC5D expression, LBL-034 consistently exhibits excellent targeted tumor cell killing effects and strong dose-dependent anti-tumor activity, showing the potential to become the best choice for similar anti-tumor drugs.
LBL-034 was approved for clinical research in China and the United States in July 2023, and an open-label, multicenter, dose-escalation/expansion Phase I/II study in patients with relapsed/refractory multiple myeloma (RRMM) was initiated in China in November 2023. Wellizbo will present preliminary clinical data from the study at the 66th Annual Meeting of the American Society of Hematology (ASH) in 2024.
https://news.yaozh.com/
By editorRead more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



