Cheng Yifan’s team reveals the activation and transmission mechanism of TGF – β signaling between cells
September 20, 2024
Source: drugdu
 299
299
The following article is from the biological world, authored by the author
Transforming growth factor beta (TGF - β) is a multifunctional cytokine that plays a critical role in processes such as development, immunity, cancer, and fibrosis. TGF - β has three different gene expression products (TGF - β 1, TGF - β 2, and TGF - β 3), all of which are expressed in an inactive (latent) form, namely L-TGF - β, and "activation" is crucial for its function.
Most therapeutic strategies targeting TGF - β do not target specific latent and/or activation mechanisms, but rather inhibit the overall TGF - β signaling pathway and exhibit significant toxicity. A deeper understanding of latency and activation may contribute to the development of better TGF - β targeted therapeutic strategies.
Traditionally, it is believed that mature TGF - β must physically dissociate from L-TGF - β 1 before signal transduction can occur. Previous research by Cheng Yifan's team has shown that integrin α v β 8 mediated TGF - β autocrine signaling transduction can occur before TGF - β 1 is released from its latent form.
On September 16, 2024, the team led by Cheng Yifan from the University of California, San Francisco published a research paper titled "Dynamic alloy drives autocrine and paracrine TGF - β signaling" in the top international academic journal Cell, revealing a novel activation mechanism of latent transforming growth factor beta (L-TGF - β) when it binds to integrin α v β 8.
This study shows that, unlike mice with TGF - β 1 deficiency (which die from tissue inflammation in early development), mice constructed through genetic engineering techniques that cannot release TGF - β 1 from L-TGF - β 1 do not have early lethal tissue inflammation and survive. Combining cryo electron microscopy technology and cell experiments, this study revealed the dynamic conformational regulation mechanism of autocrine TGF - β 1 signaling transduction, without the need for TGF - β 1 release. The binding of α v β 8 redistributes the inherent flexibility of L-TGF - β 1, allowing it to bind to receptors. Dynamic conformational regulation can explain the latency/activation mechanism of TGF - β 3 and why its function is different from that of TGF - β 1, indicating its broad applicability to other flexible cell surface receptor/ligand systems. The latency of mature TGF - β is due to the non covalent binding of its N-terminal precursor protein to TGF - β after cleavage by furin enzyme during biosynthesis. The precursor protein surrounds the mature TGF - β dimer to form a cyclic disulfide bond connected homodimer (latent associated peptide, LAP), forming L-TGF - β.
The latency of mature TGF - β is due to the non covalent binding of its N-terminal precursor protein to TGF - β after cleavage by furin enzyme during biosynthesis. The precursor protein surrounds the mature TGF - β dimer to form a cyclic disulfide bond connected homodimer (latent associated peptide, LAP), forming L-TGF - β.
Latent associated peptides (LAP) have the following four basic functions:
1) By binding the circular structure of the domain, mature TGF - β is isolated from the receptor, thereby endowing it with a latent state;
2) By binding to TGF - β microenvironment molecules (such as glycoprotein A repeat dominant protein, GARP), L-TGF - β is sealed on the matrix or cell surface, thereby stabilizing and covalently fixing L-TGF - β on the cell surface;
3) Promote correct folding and efficient secretion;
4) Binding to activating proteins, especially integrins.
Activated mature TGF - β is a homodimer connected by disulfide bonds, highly conserved in the TGF - β receptor (TGF - β R) binding region, especially mature TGF - β 1 and TGF - β 3, which have similar affinity for binding to TGF - β R (TGF - β R1/TGF - β R2). However, mice lacking TGF - β 1 or TGF - β 3 exhibit different phenotypes, which may be due to the overall low homology of LAP between TGF - β 1 and TGF - β 3, resulting in different individual mechanisms of their latent or activated states.
It is interesting that the LAP of TGF - β 1 and TGF - β 3 both contain the integrin binding motif RGDLLXXL/I, which can bind to the two integrins α v β 6 and α v β 8. This together constitutes the main functional mechanism of TGF - β 1 and TGF - β 3 in vivo. The binding of integrins ultimately leads to the activation of TGF - β and the generation of autocrine or paracrine TGF - β signaling pathways through mechanisms that have not yet been elucidated.
The structural and sequence differences between integrin α v β 6 and α v β 8 suggest that they may have different TGF - β activation mechanisms, which may lead to specific functions of TGF - β in different contexts. For α v β 6, global conformational changes transfer forces from actin cytoskeleton to L-TGF - β, disrupting LAP and allowing the release of mature TGF - β for paracrine signaling. This mechanism requires a highly conserved β 6 subunit cytoplasmic domain that binds to the actin cytoskeleton. However, it is difficult to detect the mature TGF - β 1 released from the activation mediated by α v β 8, indicating a low signaling efficiency of paracrine TGF - β 1. Therefore, there is no global conformational change in α v β 8, and the activation of TGF - β 1 mediated by α v β 8 does not require the force generated by the actin cytoskeleton, as the cytoplasmic domain of the β 8 subunit does not need to be activated and does not bind to actin.
The previous work of the research team revealed that the binding of α v β 8 enhances the binding of L-TGF - β 1, which led them to propose the hypothesis that mature TGF - β 1 can be activated without being released from latent complexes, and this hypothesis was validated in cell experiments. Therefore, the research team further speculates that the flexibility generated by binding L-TGF - β 1 to α v β 8 is sufficient to expose mature TGF - β 1 to TGF - β R for autocrine signaling without the need to release it. However, it is currently unclear how the binding of α v β 8 induces the flexibility of L-TGF - β after it is stabilized by binding to GARP in the absence of mechanical force, and whether this mechanism has physiological relevance. Because it is widely believed that the release and paracrine signaling of TGF - β are necessary for its function.
In this study, the research team first validated the physiological significance of the autocrine signal that does not release TGF - β 1. The research team designed a gene knockout mouse that only expresses TGFB1, which has a mutated furin cleavage site and cannot release TGF - β 1.
In these mice, the TGF - β signal remained intact as they were able to survive, reproduce, and avoid the lethal early tissue inflammation caused by TGF - β 1 deficiency, demonstrating that mature TGF - β can be activated, bind to its receptor, and transmit signals upon binding to its latent complex.
Next, the research team explored the mechanism of TGF - β 1 binding to TGF - β R without release, describing a dynamic conformational model - after TGF - β 1 binds to α v β 8, the local conformational entropy around the L-TGF - β RGD binding region decreases, leading to an increase in conformational entropy in the distal region of L-TGF - β/GARP, thereby exposing mature TGF - β to TGF - β R without release.
In order to support this model, the research team determined the structure of L-TGF - β 3/GARP, showing that the ground state conformation entropy of L-TGF - β 1 and L-TGF - β 3 not only determines the baseline level of integrin independent TGF - β activation, but also determines the entropy that can be used to drive integrin dependent TGF - β activation. Compared with L-TGF - β 1, the integrin mediated entropy change level in L-TGF - β 3 is higher, leading to the paracrine release of mature TGF - β 3 instead of TGF - β 1, indicating a subtype specific mechanism for autocrine and paracrine TGF - β signaling. In addition, the direction of entropy redistribution can be altered by stabilizing different flexible regions of α v β 8/L-TGF - β/GARP.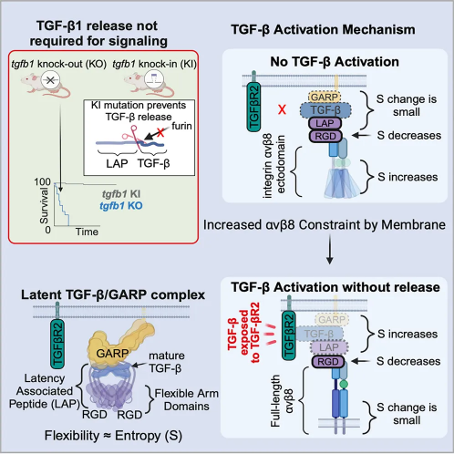 Overall, these structural and cellular studies reveal a conformational entropy redistribution mechanism based on protein dynamics across protein complexes, which is not affected by actin cytoskeleton forces and determines autocrine and paracrine TGF - β functions. These results further advance the understanding of the mechanisms underlying the latency and activation of TGF - β family members, providing a roadmap for understanding the structure of signal transduction through the protein dynamics of flexible cell surface proteins.
Overall, these structural and cellular studies reveal a conformational entropy redistribution mechanism based on protein dynamics across protein complexes, which is not affected by actin cytoskeleton forces and determines autocrine and paracrine TGF - β functions. These results further advance the understanding of the mechanisms underlying the latency and activation of TGF - β family members, providing a roadmap for understanding the structure of signal transduction through the protein dynamics of flexible cell surface proteins.
Read more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



