the dawn of treatment for multiple myeloma!
September 21, 2024
Source: drugdu
 306
306
Original Medical Overview Medical Overview September 19, 2024 08:54 Shanghai
Multiple myeloma (MM) is a blood cancer that, despite advances in treatment methods in recent years, remains an incurable disease. The traditional treatment options for MM include the combination of proteasome inhibitors (PIs), immunomodulators (IMiDs), and monoclonal antibodies (Mo Abs). Although these methods improve patient survival and progression free survival (PFS), with the emergence of treatment resistant clones, patients will eventually develop resistance to these treatments. Therefore, researchers are exploring new treatment strategies such as CAR-T cell therapy and bispecific antibodies, which activate immune T cells to kill tumor cells. Based on the review of bispecific antibodies and multiple myeloma published in the Blood Cancer Journal this month, let's talk about the design, mechanism of action, current clinical trials, and future development directions of bispecific antibodies.
01 Bispecific antibodies and therapeutic targets
Bispecific antibodies (BsAbs) are antibody constructs with two binding sites that can simultaneously bind to tumor cells and immune effector cells. Bispecific T cell engagement (BiTEs) are engineered single chain variable fragment constructs that act as dual target molecules to activate T cells and guide their killing of tumor cells. In addition, there are Tri specific antibodies (TsAb), as the name suggests, which go further by adding co stimulatory proteins to reduce T cell non reactivity or can also target additional antigens.
In order to maximize therapeutic efficacy and minimize toxicity, the design of bispecific antibodies should target antigens specific to MM cells. At present, the bispecific antibodies used to treat MM mainly target the following targets (Figure 1):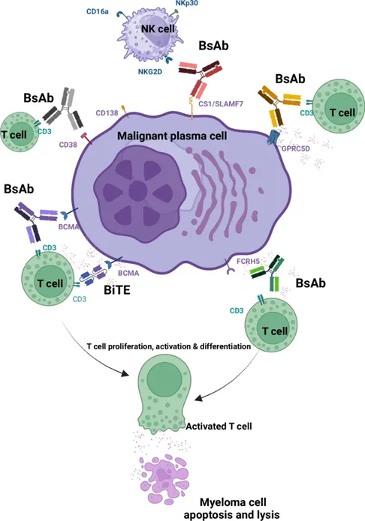 Figure 1 Bispecific antibodies and their tumor specific antigen targets against myeloma cells
Figure 1 Bispecific antibodies and their tumor specific antigen targets against myeloma cells
1. B cell membrane antigen (BCMA)
BCMA is an antigen highly expressed on both normal and malignant plasma cells, making it an ideal target for the treatment of multiple myeloma. The high expression of BCMA is associated with poor prognosis of the disease.
2. G protein coupled receptor family C group 5 member D (GPRC5D)
GPRC5D is a newly discovered immunotherapy target for multiple myeloma, and its high expression on the surface of malignant plasma cells makes it a promising therapeutic target.
3. Fc receptor homolog 5 (FcRH5)
FcRH5 is a surface facial mask protein expressed on the B-cell lineage, and its high expression on malignant plasma cells makes it a therapeutic target.
4. CD38
CD38 is an antigen highly expressed on the surface of multiple myeloma cells and is closely related to the immunosuppressive tumor microenvironment.
02 The efficacy of BsAbs in recurrent and refractory diseases
The article then summarizes the efficacy of bispecific antibodies as monotherapy in multiple clinical trials for relapsed refractory multiple myeloma (RRMM). For example, Teclisamab (a BsAb targeting BCMA) has shown significant efficacy in patients who have received at least third line treatment (Table 1).
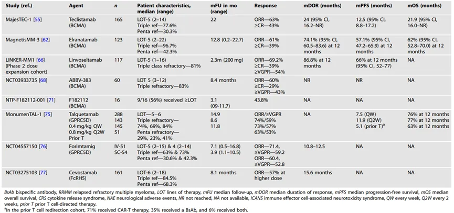 Table 1 Latest data on major clinical trials using BsAb as monotherapy for RRMM
Table 1 Latest data on major clinical trials using BsAb as monotherapy for RRMM
03 The main advantages of BsAbs
1. Innovative dual target mechanism: Dual specific antibodies can simultaneously bind to tumor cells and immune effector cells (such as T cells). Through this dual target mechanism, they can bridge and activate the immune system, especially T cells, to kill tumor cells.
2. Long induction response time: Clinical trials have shown that bispecific antibodies can induce deep and persistent tumor responses, including in patients who have undergone multi line therapy.
3. Predictable safety: Common side effects of bispecific antibody therapy include infection, decreased cell count, cytokine release syndrome (CRS), and neurological toxicity. Compared to some other types of cancer treatments, bispecific antibodies typically have predictable safety and side effects, making them easier to manage in clinical applications (Table 2).
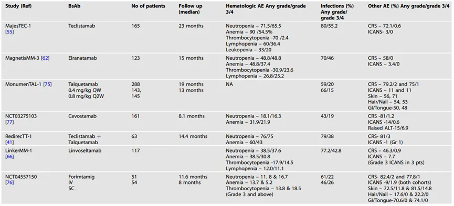 Table 2 Safety Overview and Adverse Event Summary of BsAb Clinical Trials
Table 2 Safety Overview and Adverse Event Summary of BsAb Clinical Trials
4. Ready to use: Unlike CAR-T cell therapies that require personalized manufacturing, bispecific antibodies are readily available and can be quickly used in patients without the need for long preparation and waiting times.
5. Overcoming drug resistance: MM patients may develop resistance to traditional chemotherapy drugs. Bispecific antibodies work through different mechanisms, helping to overcome or bypass certain resistance mechanisms.
6. Convenient to use: Some bispecific antibodies can be administered subcutaneously, which is more convenient than other treatments that require intravenous injection, improving the patient's treatment experience.
7. Targeting specific targets: Bispecific antibodies can be designed to target specific antigens on the surface of MM cells, such as BCMA, GPRC5D, or FcRH5, which are highly expressed on multiple myeloma cells and less expressed on normal cells, thereby improving the accuracy of treatment.
04 Drug resistance of BsAbs
Although bispecific antibodies have shown encouraging efficacy in early studies, patients may eventually develop resistance to treatment. The emergence of drug resistance is multifactorial, involving the intrinsic characteristics of tumor cells, the state of the immune system, and treatment-related factors. The main mechanisms of drug resistance are (Figure 2):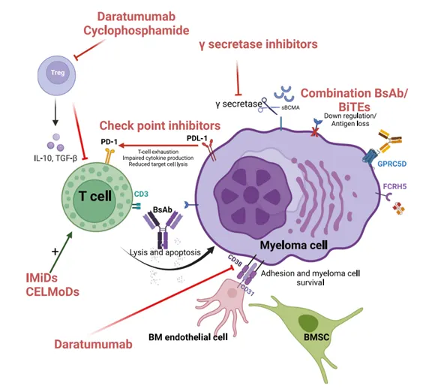 Figure 2 Resistance mechanism and mitigation strategies
Figure 2 Resistance mechanism and mitigation strategies
1. High tumor burden: Immune suppressive cells (such as regulatory T cells) and immune suppressive molecules (such as PD-L1) in the tumor microenvironment may be more active in the presence of a large number of tumors, thereby affecting the efficacy of bispecific antibodies.
2. T cell exhaustion: Continuous antigen stimulation may lead to T cell dysfunction, manifested by increased expression of inhibitory receptors on the surface of T cells, reduced cytokine production, and decreased cell killing ability.
3. Loss or downregulation of antigen expression: Over time, tumor cells may lose or reduce the expression of target antigens on their surface, such as BCMA or GPRC5D, in order to evade recognition and attack by bispecific antibodies.
4. Antigen mutation: The target antigen of tumor cells may undergo mutations, resulting in ineffective binding of bispecific antibodies.
5. Affinity and binding characteristics of bispecific antibodies: Low or high affinity for the binding of bispecific antibodies to target cells may affect the therapeutic effect.
6. Tumor Heterogeneity: The genetic and phenotypic heterogeneity of tumor cells may lead to inconsistent responses to bispecific antibodies, and some clones may be insensitive to treatment.
To overcome these resistance mechanisms, researchers are exploring various strategies, such as developing new bispecific antibodies, using other immunomodulators in combination, and targeting immunosuppressive factors in the tumor microenvironment for treatment.
05 epilogue
Bispecific antibodies have shown significant efficacy in the treatment of relapsed and refractory multiple myeloma, but this is only the beginning of a paradigm shift in treatment, and researchers are exploring moving these therapies forward to earlier treatment lines. Future research directions will focus on potential applications in early treatment, new strategies to overcome drug resistance, and combined use with other treatment methods.
Read more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



