BD receives 510(k) clearance for fingerstick blood test sample collection device
December 12, 2023
Source: drugdu
 309
309
Dive Brief
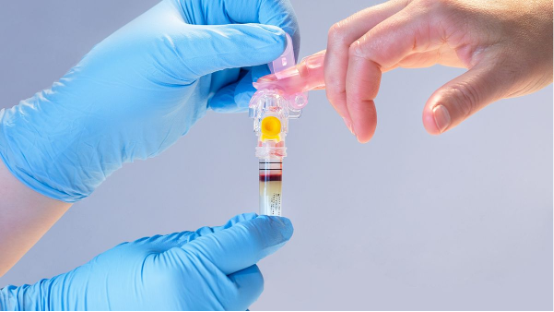
Becton Dickinson has received 510(k) clearance for a device that collects blood from a fingerstick instead of from the vein.
The MiniDraw Capillary Blood Collection System container is slightly larger than its predicate device, the BD Microtainer MAP Microtube, but is cleared for sample collection in “ancillary healthcare facilities,” positioning BD and its partner Babson Diagnostics to support blood collection from community sites such as pharmacies.
When Babson rolls out its BetterWay blood testing service next year, the device could support the collection of samples for lipid panel, selected chemistry tests, and hemoglobin and hematocrit analyses.
Dive Insight
BD began working with Babson to develop a capillary blood collection and testing system in 2019. The partners expanded their collaboration last year to support work on self-collection, mobile services and at-home collection.
Devices that enable the collection of capillary, rather than venous, blood for tests of hemoglobin and hematocrit are already on the market. The predicate device cited in the 510(k) filing was cleared by the Food and Drug Administration in 2010. Clinical laboratories use the BD Microtainer MAP Microtube to test for hematology analytes and lead in capillary samples.
BD and Babson’s innovation is to create a blood collection device that can be used by trained healthcare workers outside of standalone labs, eliminating the need for a phlebotomist to collect blood from a vein. That advance, coupled with the scope of the 510(k), supports the collection of blood at more sites.
“Because the BD MiniDraw Collection System enables blood collection at non-traditional sites that may be more convenient — like your local pharmacy rather than a standalone lab — we can expand health equity and access, and make it easier for patients to get the blood tests they need,” Dave Hickey, executive vice president and president of Life Sciences for BD, said in a statement.
The focus on enabling people to provide small volumes of blood at community locations is reminiscent of the ambitions of Theranos, the startup that pitched itself as a revolutionary advance in testing before the truth about its technology came out and charges of fraud were prosecuted. However, the BD MiniDraw Capillary Blood Collection System is a different proposition.
Theranos claimed to run 70 different laboratory tests from a single draw. BD’s device collects more blood for use on a shorter list of tests. Theranos’ “nanotainer” collection device was also never cleared by the FDA.
Source:
https://www.medtechdive.com/news/bd-fda-clearance-fingerstick-blood-test-collection/701965/
Read more on
- Anglikang Adenosylcobalamin Capsules Obtain Drug Registration Certificate March 6, 2026
- Two Minoxidil Topical Solution Applications from its Subsidiary Approved March 6, 2026
- Zhejiang Medicine’s ARX305 Initiates Phase II Clinical Trial March 6, 2026
- Indacaterol Mometasone Inhalation Powder Clinical Trial Approved for Asthma Treatment March 6, 2026
- Harsco Pharmaceutical’s innovative drug HSK50042 tablets receive clinical approval for a new indication March 6, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



