Allakos Skin Drug Fails 2 Mid-Stage Tests, Sparks Restructuring to Cut 50% of Staff
January 18, 2024
Source: drugdu
 314
314
Allakos’s lirentelimab did not beat a placebo in separate Phase 2 tests in atopic dermatitis and spontaneous chronic urticaria. A restructuring now turns the biotech’s focus to an early-stage drug candidate also designed to treat inflammation.
By FRANK VINLUAN
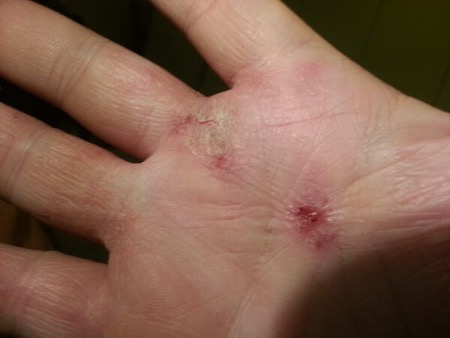 The lead therapeutic candidate of Allakos has failed in two mid-stage clinical trials for inflammatory skin disorders, leading the biotech to halt work on the drug. Allakos is shifting its focus to an earlier-stage program in its pipeline but will do so with fewer staff. A corporate shakeup is shaving about half of the company’s workforce.
The lead therapeutic candidate of Allakos has failed in two mid-stage clinical trials for inflammatory skin disorders, leading the biotech to halt work on the drug. Allakos is shifting its focus to an earlier-stage program in its pipeline but will do so with fewer staff. A corporate shakeup is shaving about half of the company’s workforce.
San Carlos, California-based Allakos aims to treat allergic, inflammatory, and proliferative diseases with antibodies that target receptors on cells that create immune responses in the body. By activating inhibitory receptors, these drugs are intended to stop inflammation. Its most advanced drug candidate was lirentelimab, which was designed to target an inhibitory receptor on mast cells and eosinophils, two types of white blood cells that play key roles in inflammatory responses.
The preliminary Phase 2 data announced Tuesday are from studies evaluating lirentelimab in atopic dermatitis, which leads to red and flaky patches on the skin, and chronic spontaneous urticaria, which causes hives. In both studies, results showed reductions in levels of eosinophils in the treatment arms compared to the placebo groups. But the main goals of both studies were to show changes according to scoring assessments used to evaluate the severity of symptoms in the respective diseases. While the Allakos drug led to improvement in scores, the results were not enough to beat the placebo arms.
“We are disappointed that these trials did not meet their primary endpoint, particularly given the need for new treatment options for patients with these severe diseases,” Chief Medical Officer Craig Paterson said in a prepared statement. “Given that neither trial met its primary endpoint, we have decided to not pursue further clinical development of lirentelimab.”
Allakos’s hopes now ride on AK006, an antibody that targets a different inhibitory receptor than lirentelimab. A Phase 1 program is underway, dosing healthy volunteers with an intravenous version of the drug. In the current quarter, the company expects to complete dosing of the IV cohort and begin dosing in a group given a subcutaneously injected version. Initial results from the IV cohort are expected in the second quarter of this year. Allakos then plans to proceed to a placebo-controlled Phase 1 test of the IV version of AK006 in patients with chronic spontaneous urticaria. Preliminary data from this study are expected by the end of the year.
In a note sent to investors Tuesday, William Blair analyst Tim Lugo said AK006 represents Allakos’s third and likely final shot at hitting mast cells. But he added that this antibody inhibits mast cell activation via multiple stimuli, which offers deeper and broader mast cell inhibition than lirentelimab.
Allakos reported having about $171 million in cash, cash equivalents, and investments at the end of the third quarter of 2023. Closing out the lirentelimab program and severance costs will total about $30 million, Allakos said. With the restructuring cutting about 50% of its workforce, the company expects its cash will last into mid-2026. The company headcount at the end of 2022 was 123 full-time employees, according to Allakos’s annual report.
Photo by Flickr user Oregon State University via a Creative Commons license
By editorRead more on
- Marketing application for HSK39297 tablets, an innovative drug for paroxysmal nocturnal hemoglobinuria, has been accepted March 9, 2026
- Clinical trial application for olopatadine-mometasone nasal spray approved by NMPA March 9, 2026
- Superior to standard therapy! Johnson & Johnson’s blockbuster new drug combination therapy receives FDA approval March 9, 2026
- For the first time, a domestically produced ultra-long-acting TSLP bispecific antibody has been approved for clinical trials. March 9, 2026
- With Hengrui entering the fray, can Mingrui hold its ground? March 9, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



