Longer Treatment Leads to More Liver Benefit
March 6, 2024
Source: drugdu
 337
337
Akero Therapeutics’ preliminary Phase 2b data show that treatment with its MASH drug, efruxifermin, continued to distance itself from a placebo measured at nearly two years of treatment. The latest Akero data build on six-month results reported in 2022.
By FRANK VINLUAN
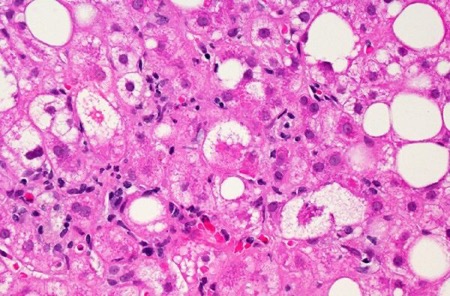 Longer treatment with an Akero Therapeutics drug in development for the liver disease MASH led to better results, including improvement in fibrosis, the liver scarring that is a hallmark of the chronic disorder.
Longer treatment with an Akero Therapeutics drug in development for the liver disease MASH led to better results, including improvement in fibrosis, the liver scarring that is a hallmark of the chronic disorder.
Metabolic dysfunction-associated steatohepatitis, or MASH, causes deteriorating liver function that can reach the point of requiring an organ transplant. The fibrosis that develops with MASH is classified in four stages. Stage 4, the most severe, is liver cirrhosis.
On Monday, South San Francisco-based Akero reported preliminary results at 96 weeks showing that 75% of patients treated with the highest dose of its drug, efruxifermin, showed improvement of at least one stage of fibrosis without worsening of MASH. In the group of patients who received the low dose, 46% of patients met this mark. By comparison, 24% of study participants in the placebo group met that main clinical trial goal.
The latest Phase 2b results improve on data reported in 2022. At that time, this study met the main clinical trial goal with 41% of patients in the high dose group achieving at least one stage of improvement in fibrosis at six months. In the low dose group, 39% of patients met that goal. In the placebo arm, 24% of study participants met that mark.
Efruxifermin is a fusion protein that serves as an analog of fibroblast growth factor 21, or FGF21. This hormone found in the body protects against cellular stress and also regulates metabolism. But compared with native FGF21, the Akero drug is engineered to have a longer half-life. Efruxifermin is administered as a once-weekly injection.
Akero said is drug was well-tolerated by patients. No deaths were reported in the Phase 2 study. A total of 15 serious adverse events were reported, which the company said was balanced across the dose groups. In the two treatment arms, the most frequent adverse events were grade 1 or 2 gastrointestinal complications, such as diarrhea, nausea, and increased appetite. But Akero said these problems were transient.
The latest clinical trial results for the Akero drug are for patients who were pre-cirrhotic—their fibrosis was classified as either stage 2 or 3. Last October, Akero reported results from a separate Phase 2b test in MASH patients with stage 4 fibrosis showing the drug did not achieve the main goal of improving fibrosis after 36 weeks. While the company reported numerical improvements for both doses of efruxifermin, those marks were not enough to distance the drug from a placebo. At the time, the company said it believed longer exposure to the drug has the potential to show higher rates of improvement.
The company expects 96-week results from the Phase 2b test in cirrhotic patients will become available in the first quarter of 2025. But in the meantime, the results announced Monday in pre-cirrhotic MASH patients suggest longer treatment with the Akero drug benefits patients in earlier stages of their disease. A Phase 3 clinical trial in pre-cirrhotic MASH patients is currently enrolling patients.
“Today’s results show that longer exposure to [efruxifermin] has the potential to yield sustained fibrosis improvement as well as widening anti-fibrotic treatment responses across the treated patient populations,” Akero President and CEO Andrew Cheng said in a prepared statement.
Public domain image by the Flickr user NIH Image Gallery
Read more on
- Gusekirumab Injection Accepted by CDE, Multiple Pipelines Advancing Simultaneously March 4, 2026
- Yifan Pharmaceutical’s teriparatide injection has been accepted by the CDE (Center for Drug Evaluation), adding a new domestic player to the osteoporosis treatment field March 4, 2026
- //news.yaozh.com/archive/47318.html PD-1 sales surge March 4, 2026
- A major breakthrough! Roche’s oral BTK inhibitor achieves its third Phase III clinical trial victory, a game-changer in the multi-billion dollar MS (manufactured pharmaceuticals) market. March 4, 2026
- GB19 Injection Approved for Clinical Trials of Cutaneous Lupus Erythematosus March 4, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



