Abbott ramps up over-the-counter CGM race with new sensors
June 12, 2024
Source: drugdu
 428
428
 Dive Brief
Dive Brief
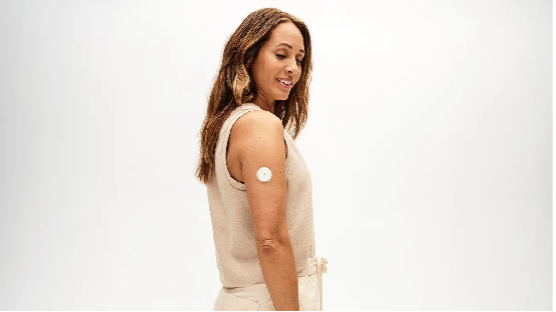
Abbott said Monday it will launch two over-the-counter continuous glucose monitors after receiving clearance from the Food and Drug Administration.
One product is the company’s Lingo device, sold as a wellness product for people who do not have diabetes. The other is Abbott’s new Libre Rio device, which is intended for adults with Type 2 diabetes who do not use insulin, posing a direct challenge to Dexcom’s Stelo device.
After Lingo was cleared last week, RBC Capital Markets analyst Shagun Singh wrote the over-the-counter nod could offer a more than $1 billion sales opportunity for Abbott.
Dive Insight
Abbott had discussed plans to bring Lingo to the U.S. after releasing it in the U.K. last year. The device, which can be worn on the upper arm for 14 days, tracks glucose to help people understand how their bodies react to different foods, exercise and stressors. Abbott received FDA clearance on May 29 to sell its Lingo system over the counter.
RBC Capital Markets’ Singh wrote that the Lingo clearance could expand Abbott’s total addressable market to 24.5 million people with Type 2 diabetes who don’t take insulin and 96 million people in the U.S. who have prediabetes.
On Monday, Abbott unveiled another product: Libre Rio, a CGM for adults with Type 2 diabetes who do not use insulin. The FDA cleared the device on Friday. Abbott kept the project under wraps, but is now poised to compete with Dexcom, which received clearance in March for the first over-the-counter CGM, intended for people with diabetes who don’t take insulin.
Rio is the first over-the-counter CGM with a measurement range of 40 to 400 mg/dL, allowing for measurement of extremely low or high glucose events, Abbott said.
“There is no one-size-fits all approach for glucose monitoring, which is why we’ve designed different products for different people — all based on the same world-leading biowearable technology,” Lisa Earnhardt, group president of Abbott’s medical devices business, said in a statement. “People living with diabetes need certain features like tracking medications or sharing data with a healthcare provider. People without diabetes need different features to manage their metabolic health, including personalized coaching to promote actionable lifestyle changes.”
Abbott also continues to develop a sensor that can measure both glucose and ketone levels, which could help people avoid diabetic ketoacidosis, a complication of Type 1 diabetes.
Source:
https://www.medtechdive.com/news/abbott-over-the-counter-cgm-rio-lingo-clearance/718451/
Read more on
- China Sino Biopharmaceutical Signs Exclusive Licensing Agreement with Sanofi for Rofalcitinib March 4, 2026
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



