1st domestic product! One medical device has obtained CE MDR certification
February 27, 2025
Source: drugdu
 306
306
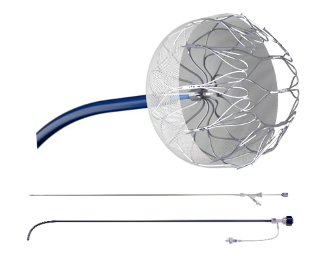 Recently, MicroPort Xintong Medical Technology Co., Ltd. (hereinafter referred to as Xintong Medical) announced that its subsidiary Shanghai Zuoxin Medical Technology Co., Ltd. has developed AnchorMan ® The left atrial appendage occlusion system has successfully obtained the EU CE MDR certification, becoming the first left atrial appendage occlusion system in China to receive this certification.
Recently, MicroPort Xintong Medical Technology Co., Ltd. (hereinafter referred to as Xintong Medical) announced that its subsidiary Shanghai Zuoxin Medical Technology Co., Ltd. has developed AnchorMan ® The left atrial appendage occlusion system has successfully obtained the EU CE MDR certification, becoming the first left atrial appendage occlusion system in China to receive this certification.
In addition, the AnchorMan used in conjunction with it ® The left atrial appendage guidance system was also approved for market by the European Union during the same period.
The first and only product in the country
In recent years, with the further growth of the aging population, the incidence rate of atrial fibrillation has increased year by year. Among them, thromboembolism is the most common complication of atrial fibrillation, with the highest incidence and greatest harm of stroke. The stroke rate of atrial fibrillation patients is five times higher than that of the general population.
Moreover, the left atrial appendage is the main site of atrial fibrillation thrombus formation. Left atrial appendage occlusion (LAAC) is an interventional surgical procedure that involves puncturing the femoral vein and delivering a disc or plug occluder through the femoral vein to the left atrial appendage to expand and block it, thereby preventing thrombus formation in the left atrial appendage and achieving the goal of reducing embolic events. LAAC surgery has definite therapeutic effects, minimal trauma, no obvious pain, fast recovery, safety, and high success rate. It can eliminate patients' dependence on long-term oral anticoagulant therapy and provide new treatment options for patients who cannot receive anticoagulant therapy for a long time, are unwilling to take anticoagulant therapy, and are at high risk of bleeding.
Compared to traditional open and closed left atrial appendage occluders, AnchorMan ® The left atrial appendage occluder system combines the advantages of both, forming a semi closed structure with 12 "3D folding" units at the tail and a mesh frame, solving the clinical pain point of traditional internal plug occluder sheaths that must penetrate deep into the atrial appendage, and achieving stable anchoring of the instrument; The distal end of the instrument is rounded and soft, which can reduce damage to the atrial appendage tissue; The dense nickel titanium alloy mesh design makes it better conform to the ear and improves the sealing effect; In addition, it has two release methods, push and retract, providing surgeons with more choices.
Thanks to the unique design of the product, the results of its pre-market clinical study on SAFE Protect were also excellent. The product reached the primary endpoint and secondary efficacy endpoint of the study within one year, with a low incidence of stroke, systemic embolism, major bleeding, transient ischemic attack (TIA), device related complications, and device relative thrombosis (DRT) in patients. Its significant high clinical success rate and low clinical adverse events one year later are attributed to its ingenious research and development design.
It is worth mentioning that the product was successfully approved for market by the National Medical Products Administration (NMPA) of China in January 2024, becoming the first and currently the only semi closed plug type left atrial appendage occluder product approved in China.
Multiple heavyweight products approved
Xintong Medical is a subsidiary of Minimally Invasive Medical Science Co., Ltd. It originated from its valve pre research project established in 2009, officially established in 2015, and listed on the main board of the Hong Kong Stock Exchange in February 2021. Since its establishment, Xintong Medical has focused on the research and commercialization of innovative catheterization and surgical solutions in the field of structural heart disease.
At present, Xintong Medical's product pipeline is also quite rich, divided into five major fields: aortic valve products, mitral valve products, tricuspid valve products, surgical valve products, and surgical supporting products. Various products are in different research and development stages, and Xintong Medical is actively expanding and enriching its research pipeline.
In its product pipeline, the VitaFlow Liberty developed by it ® It is the first and currently the only electric retrievable transcatheter aortic valve system in China, with excellent performance such as high radial support, better coaxial release, and effective reduction of postoperative paravalvular leakage.
VitaFlow Liberty ® Inherited VitaFlow from Xintong Medical ® The advantages of transcatheter aortic valve and delivery system in valve design include the use of a mixed density self expanding stent, bovine pericardial valve leaflets, and a high bilayer PET skirt design, which have excellent performance such as high radial support, better coaxial release, and effective reduction of postoperative perivalvular leakage and reflux. In addition, its breakthrough upgraded conveying system features a unique double helix innovative structure, which ensures fast, stable, and precise release and recovery while achieving the flexibility of the conveying system and 360 ° bending function of the valve segment.
In addition, its VitaFlow Liberty ® The Flex Transcatheter Aortic Valve Recyclable and Controllable Bend Delivery System was approved for market by NMPA in December 2024. This product is currently the only "true" coaxial controlled bend self expanding TAV delivery system and the only electric retrievable TAV delivery system in the world.
It is reported that this product has multiple differentiated advantages:
Unique X3 Flexis ™ 3D bending control function, which uses snake bone traction to achieve smoother and safer arch and valve crossing through 3D bending design, bringing better coaxiality;
The curved segment is shorter and closer to the stent, which is more suitable for the anatomical structure of the patient's aorta;
Its unique capsule segment tube bending technology can keep the valve coaxial during release, making implantation more stable and accurate;
The commissural alignment function achieves the alignment of the artificial valve with the patient's native valve during valve release, protecting the coronary pathway and reserving space for future coronary interventions;
The overall delivery system adopts composite flexible pipes, which are more flexible and reduce the risk of vascular complications;
The intelligent limit switch design enables intelligent braking during the release process and comes with sound and light reminders, making the operator's operation more reassuring.
Conclusion
The two heavyweight products that have obtained CE MDR certification this time are of great significance to Xintong Medical, as they have successfully opened the door to the European market in the field of stroke prevention for atrial fibrillation patients. At present, the "gold content" of domestic medical devices is becoming increasingly high, and China’s medical devices are continuously shining with new brilliance.
Source: https://news.yaozh.com/archive/45044.html
By editorRead more on
- API Is Not Just a Cost – It’s Your Ticket to Global Markets March 3, 2026
- Rovaxitinib approved for marketing, filling the demand for myelofibrosis treatment March 2, 2026
- Warrant Pharmaceuticals’ active pharmaceutical ingredient receives Brazil’s first official GMP certification March 2, 2026
- Merck’s New Story March 2, 2026
- Rongchang Biotechnology has turned a profit! March 2, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



