1.5 billion US dollars! Capricor Therapeutics partners with Nippon Pharmaceutical Co., Ltd
September 19, 2024
Source: drugdu
 295
295
Disclaimer: Due to limited proficiency, errors are inevitable, or some information may not be timely. Please feel free to leave a message to indicate. This article only provides an introduction to medical and health-related drugs, and does not recommend treatment plans (if applicable); This article does not constitute any investment advice.
On September 17, 2024, Capricor Therapeutics, a biotechnology company developing transformed cell and exosome therapies for the treatment of rare diseases, announced that it had signed a binding term sheet with Nippon Pharma.
The terms state that Nippon Pharma will commercialize and distribute Capricor's main asset, deramiocel, in Europe for the treatment of Duchenne muscular dystrophy (DMD). Capricor will receive a $15 million equity investment at a 20% premium and a $20 million advance payment upon signing the final agreement. The potential milestone amount is as high as $715 million, with product revenue accounting for double digits. The total potential milestone for the comprehensive distribution agreement is currently approximately $1.5 billion.
Dr. Linda Marb á n, CEO of Capricor, said, "Our expansion of cooperation with Nippon Shinyaku in Europe marks a critical moment for Capricor as we work together to bring deramiocel to DMD patients worldwide. With the advance payment and equity investment, we will be able to extend our development path until 2026 and potentially obtain deramiocel approval in the United States and other regions. In addition, these funds will provide necessary funding for commercial launch preparation, production scale expansion, and product development in Europe, as we anticipate high global demand for deramiocel
Toru Nakai, President of Nippon Pharma, said, "We look forward to expanding the commercial footprint of deramiocel globally. This collaboration will enable us to continue investing in the DMD franchise of Nippon Pharma and potentially provide life changing treatments for patients in need
About Deramiocel (CAP-1002)
Deramiocel is composed of allogeneic cardiac derived cells (CDC), which are a type of stromal cell. Preclinical and clinical studies have shown that they play a powerful role in immune regulation, anti fibrosis, and regeneration in muscular dystrophy and heart failure. CDC works by secreting extracellular vesicles called exosomes, which target macrophages and alter their expression profile, resulting in a healing phenotype rather than a pro-inflammatory phenotype. The CDC has been the subject of over 100 peer-reviewed scientific publications and has been used in over 200 human subjects in multiple clinical trials. Deramiocel, which is used to treat DMD, has obtained orphan drug status, and its regulatory pathway is supported by RMAT (designated as an advanced therapy in regenerative medicine). In addition, if Capricor obtains FDA approval to use deramiocel for the treatment of DMD, Capricor will be eligible for a priority review voucher (PRV) as it has previously been designated for rare pediatric diseases.
About Capricor Therapeutics
Capricor Therapeutics, Inc. (NASDAQ: CAPR) is a biotechnology company dedicated to advancing translational cell and exosome therapies to redefine the therapeutic prospects of rare diseases. The forefront of our innovation is our main candidate product deramiocel (CAP-1002), an allogeneic cardiac derived cell therapy. Extensive preclinical and clinical studies have shown that deramiocel has immunomodulatory, anti fibrotic, and regenerative effects specifically targeting muscular dystrophy and heart disease. Deramiocel is currently advancing Phase 3 clinical development for the treatment of Duchenne muscular dystrophy. Capricor also utilizes the power of its exosome technology, utilizing its proprietary StealthX ™ The platform focuses on preclinical development, with a particular emphasis on targeted delivery of vaccines, oligonucleotides, proteins, and small molecule therapies to potentially treat and prevent various diseases. At Capricor, we are committed to breaking the boundaries of possibilities and opening up a transformative path of healing for those in need.
Capricor R&D pipeline
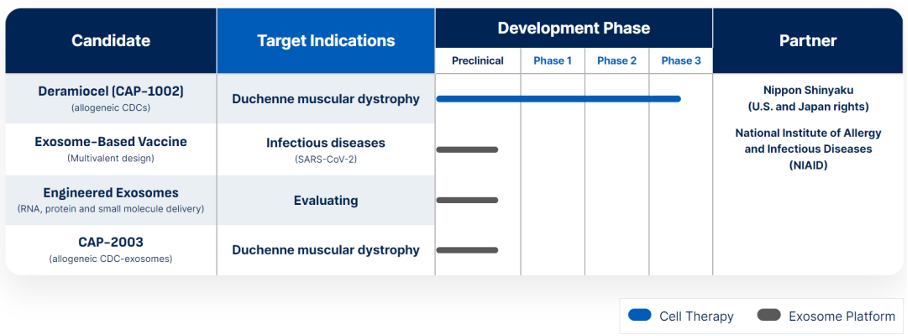
Information source: Capricor Therapeutics official website
By editorRead more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



