Ddu College:Pharmacovigilance
August 24, 2021
Source: Ddu
 2,282
2,282

Background
Pharmacovigilance consists of a combination of the wording pharmaco- and vigilance: Pharmakon (Latin means "medicine, pharmacy"), vigilare (Latin means "alert, vigilant").In 1974, French first raised the word “pharmacovigilance”, since then its concept and meaning are constantly evolving and developing. Today's pharmacovigilance does not simply represent a monitoring report of adverse drug reactions, but it focuses on adverse drug reactions or ADR particularly.A recognized explanation for adverse drug reactions is for those harmful and undesired drug reactions that occur during the prevention, diagnosis or treatment of a disease or the regulation of physiological functions at normal use doses.
Pharmacovigilance
A discipline that collects, monitors, researches, and evaluates information about health care providers and patients regarding to the adverse drug reactions , biological products, herbs, and traditional medicine.
Main feature
"Pharmacovigilance" belongs to the scope of the public health care system, a macroscopic and large-scale implementation system which is generally operated by state or regional government agencies to implement supervision and enforcement.The difference between "Pharmacovigilance" and "Monitoring for adverse drug reaction":The pharmacovigilance covers the entire process from drug research and development to marketing, while adverse drug reaction monitoring refers only to the monitoring of drugs after they are marketed.The monitoring targets are different: the adverse drug reaction monitoring targets the qualified drugs, while the pharmacovigilance refers to drugs other than qualified drugs such as drugs below the legal standards, drugs and compounds or interactions between drugs and foods and so on.The work contents are different: pharmacovigilance duty range includes monitoring of adverse drug reactions and other tasks which are finding the medication errors, checking the lack of efficacy, using drugs for indications without adequate scientific evidence or approval, reporting acute and chronic poisoning cases, evaluating drug-related mortality and detecting drug abuse and misuse.
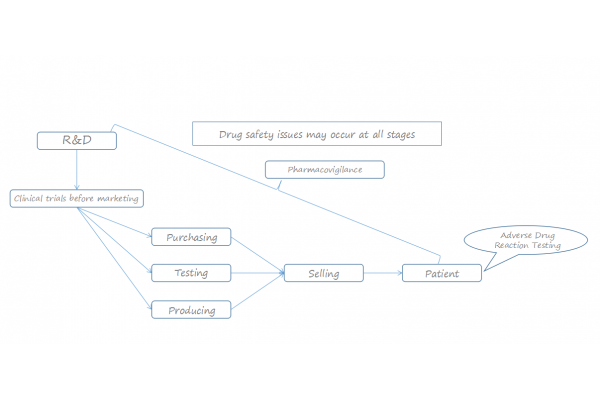 Domestic development status
Domestic development status
Late beginning with a slow developing speed is the current reality of China's pharmacovigilance development.At present, the national pharmacovigilance process is mainly constructed, managed, and implemented by China Food and Drug Administration (CFDA) and the National Center for Adverse Drug Reaction Monitoring."Drug Adverse Reaction Reporting and Monitoring Management Measures" - 2011 Edition is the only "pharmacovigilance" in China that can refer to relevant policies and regulations.The official authoritative journals for domestic pharmacovigilance information are "Chinese pharmacovigilance" and "Adverse Drug Reaction Journal", which will update the latest pharmacovigilance information inside and outside the country.
By Ddu
Read more on
- 4.1 billion US dollars! Thermo Fisher Scientific acquires Solventum’s purification and filtration business June 5, 2025
- How Can Pharmaceutical Exporters Secure Their First Overseas Distributor?— A Practical Guide from Sourcing to Evaluation and Negotiation May 16, 2025
- Career Development Guide for API and Pharmaceutical Intermediate Sales Professionals April 8, 2025
- How Can AI Empower Medical B2B Trade? A Must-Try Industry Assistant March 17, 2025
- Revolutionizing Medical B2B Trade with AI-Powered Intelligence February 28, 2025
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



