-
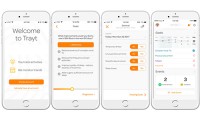
New Trayt’s app tracks neurodevelopmental disorders symptoms
- Source: Jophy Joseph
- 1,233
- May 3, 2018
-
Tisagenlecleucel Granted FDA Approval for Large B-Cell Lymphoma
- Source: targetedonc
- 3,888
- May 3, 2018
-
iBeat’s $5.5M funding paves way for summer launch of cardio wearable
- Source: MobiHealthNews
- 878
- May 3, 2018
-
Nokia is selling its digital health business back to the co-founder of Withings
- Source: The Verge
- 757
- May 3, 2018
-
AI-powered doctor assistant Suki lands $20M in funding
- Source: MobiHealthNews
- 981
- May 2, 2018
-
Fitbit will use Google Cloud to make its data available to doctors
- Source: Tech Crunch
- 1,137
- May 2, 2018
-
Manufacturing hiccup in China trips up Sanofi’s vaccine sales momentum
- Source: fiercepharma
- 845
- May 2, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.