-
Drugmakers submit counteroffers to US Medicare pricing negotiations
- Source: drugdu
- 371
- March 8, 2024
-
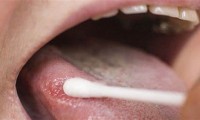
Noninvasive, Low-Cost Test Accurately Diagnoses Oral Cancer in 30 Minutes
- Source: drugdu
- 346
- March 7, 2024
-
Simple Blood Test Measures Repetitive DNA for Early Cancer Detection
- Source: drugdu
- 542
- March 7, 2024
-
FDA Approves AbbVie’s Juvederm Voluma XC for the Treatment of Moderate to Severe Temple Hollowing
- Source: drugdu
- 418
- March 7, 2024
-
FDA Approves Sandoz Biosimilars for Two Blockbuster Amgen Bone Drugs
- Source: drugdu
- 325
- March 7, 2024
-
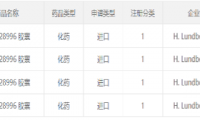
Lundbeck Pharmaceuticals’ “Anti-Parkinson’s Category 1 Chemical Drug” Submits Clinical Application in China
- Source: drugdu
- 591
- March 7, 2024
-
FDA Clears First Over-the-Counter Continuous Glucose Monitor
- Source: drugdu
- 275
- March 7, 2024
-
Sinopharm and the University of Macau Signed Strategic Cooperation Framework Agreement
- Source: drugdu
- 388
- March 7, 2024
-
Shuangcheng Pharmaceuticals Octreotide Acetate Injection Obtained Certificate of Drug Registration
- Source: drugdu
- 384
- March 7, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.