-
CellVoyant secures £7.6 million AI stem cell boost
- Source: drugdu
- 410
- April 7, 2024
-
Aston University receives £10m for membrane institute
- Source: drugdu
- 362
- April 7, 2024
-
Disco tunes into new cancer drug targets
- Source: drugdu
- 421
- April 7, 2024
-
Poolbeg’s cytokine release syndrome drug shows potential
- Source: drugdu
- 362
- April 7, 2024
-
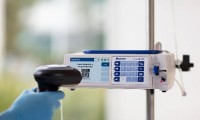
Baxter receives FDA clearance for delayed Novum IQ infusion pump
- Source: drugdu
- 423
- April 5, 2024
-
Abbott nets FDA approval for Triclip
- Source: drugdu
- 360
- April 5, 2024
-
Alebund receives ODD for investigational drug AP303
- Source: drugdu
- 461
- April 5, 2024
-
Bavarian Nordic expands access to mpox vaccine in US
- Source: drugdu
- 373
- April 5, 2024
-
Xilio fires 21% of its workforce and reprioritises pipeline
- Source: drugdu
- 353
- April 5, 2024
-
Cellenkos links with Mount Sinai to explore CK0804 for myelofibrosis
- Source: drugdu
- 289
- April 5, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.