Lumendi Acquires CE-Mark on DiLumen EIP Device
July 23, 2018
Source: The Verdict
 1,311
1,311
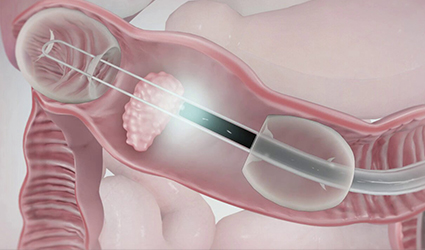
UK-based medical device manufacturer, Lumendi obtained the European CE-Mark for its DiLumen Endolumenal Interventional Platform (EIP) which is used as an endoscope accessory.
The device, designed to enable absolute positioning of a conventional endoscope in the large intestine and enhance optical visualization, diagnosis and treatment, is a non-sterile, single-use, close-fitting sleeve.
Lumendi is currently developing a distribution network to commercially launch the device in the European Union (EU) market, post its successful achievement of acquiring the CE-Mark. Distributors for Italian and British regions have already been established, said the company.
Lumendi CEO Peter Johann said: “Receiving approval to apply the CE-Mark in Europe is critical to Lumendi’s overall global strategy to open markets around the world to continue improving endoscopic interventions and migrating many gastrointestinal surgeries to less invasive endoluminal procedures.”
“To make the DiLumen EIP platform available across the EU, we are building a team to support our distributors in Europe in both marketing and clinical application.”
Lumendi aims at shifting various gastrointestinal surgeries to endoluminal procedures. The endoluminal approach comes with a number of benefits such as minimizing general anesthesia requirements, enabling an incisionless approach, diminishing complications of open or laparoscopic surgery, shortening hospital stays and cutting down expenses.
DiLumen EIP comprises of two balloons which, when deployed and inflated, stabilizes the area between them. This area is called the therapeutic zone (TZ) which also allows insufflation and manipulation of the tissue.
By DduRead more on
- 【EXPERT Q&A】What are the regulations and requirements for exporting medical devices to the European Union? September 5, 2023
- 【EXPERT Q&A】What is the procedure for registering medical devices for the Russian market? August 22, 2023
- Things to Know before Buying Newborn Baby Incubators March 31, 2022
- Portable Nebulizer Machine September 10, 2018
- PHYSIOTHERAPY TABLE September 7, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



