Endomag Breast Cancer Device Gains FDA Approval
August 1, 2018
Source: The Verdict
 729
729
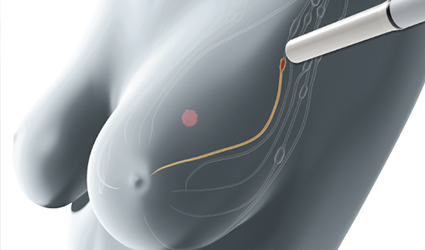
Endomag received premarket approval from the FDA for Magtrace, the novel non-radioactive dual-tracer for lymphatic plotting in breast cancer patients which facilitates the implementation of magnetic detection during sentinel lymph node biopsy procedures to spot sentinel lymph nodes for surgical excision.
The University of California, San Francisco (UCSF) professor of surgery Michael Alvarado, the principal investigator of the US Magtrace trial, said: “We’ve been watching this technology become established in Europe over the past few years, and have been eagerly awaiting its availability in the US.
“After 18 months of using Endomag’s Sentimag platform with their Magseed marker for lesion localization, we’re really excited to add the sentinel node biopsy capability with Magtrace. Being able to carry out both seed localization and sentinel node biopsy in one box made this the only option for us. Magtrace and Magseed not only help to reduce the hospital staff and patients from exposure to radioactivity, they also offer us flexibility and so many more options when deciding on how to approach our breast cancer patients.”
The gathering of the Magtrace in these nodes helps surgeons to precisely mark them for excision, leaving other nodes in the region intact. This is imperative for labeling the tumor stage and selecting the best treatment protocol for the patient.
The device is a dual-tracer including a magnetic fluid with iron oxide particles that deliberate the most probable route of cancer cells when they metastasize throughout the body. The fluid can either be injected during the surgery or up to a week before surgery as the trace is minute enough to travel swiftly through breast tissue but large enough to be filtered via the earliest draining of sentinel lymph nodes.
By DduRead more on
- 【EXPERT Q&A】What are the regulations and requirements for exporting medical devices to the European Union? September 5, 2023
- 【EXPERT Q&A】What is the procedure for registering medical devices for the Russian market? August 22, 2023
- Things to Know before Buying Newborn Baby Incubators March 31, 2022
- CRUK researchers develop new early-stage rectal cancer treatment December 12, 2020
- AZ’s Farxiga Gets FDA Priority Review For Heart Failure January 8, 2020
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



