New Regulations for Drug Barcodes in Pakistan
April 9, 2018
Source: Ddu
 2,902
2,902

In June, 2017, the Drug Regulatory Authority of Pakistan (DRAP) issued Statutory Notifications (S.R.O) 470 (I)/2017, a new regulation stating that imported and exported drugs should realize serialization and meet the barcode label requirements for the product track and trace system in six months.
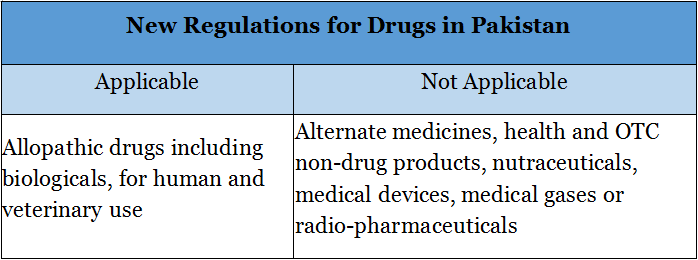
Ddu, the leading global pharmaceutical & medical device B2B online platform, investigated and found that according to the notification, the new regulations will be applicable to allopathic drugs including biologicals, for human and veterinary use only and does not apply to alternative medicines, health and OTC non-drug products, nutraceuticals, medical devices, medical gases or radio-pharmaceuticals.
The new regulations are one of the measures taken by the Pakistani government in an attempt to crack down on counterfeit drugs. It was been stipulated that all drugs manufactured for the domestic market, both exported and imported, must be labeled with machine-readable barcodes to facilitate identification, tracking and tracing. Additionally, if any imported drugs do not conform to specifications, the company importing the drug in question must make arrangements with a local licensed facilities to ensure all relevant information is available and meets the stipulated standards before the drug is released onto the Pakistani market.
The machine-readable 2D barcode, also named global trade identification number (GTIN), is composed of 14 characters and includes information such as expiration date, batch lot and product features (name, dose and package size of the drug).

Pharmaceutical manufacturers need to submit product and company information directly to the online database of the Drug Regulatory Information System (DRIS), coordinated by DRAP, as well as maintain their own product barcode information database. DRAP will also develop a complete tracking system in accordance with international practices.
At the end of 2016, soon after the promulgation of relevant laws and regulations requiring drug registration documents to be submitted in CTD format, new regulations followed, proving that DRAP is keeping abreast of the ever-changing situations in the European and American regulatory markets.
Saira Afzal Tarar, minister of the Pakistan National Health Service, indicated that these new regulations will vigorously promote the elimination of counterfeit drugs.
Support for the new regulations comes from a multitude of different places with varied opinions on how to implement them. Haroon Qassim, former chairman of the Pakistan Pharmaceutical Manufacturers Association, said: "Serialization is a good and radical idea, by doing this we are one step ahead of the USA and Europe.” The Pakistan Pharmaceutical Manufacturers Association (PPMA) did however, urge DRAP to reconsider the measures, claiming that six months is too short a time period to put everything in place, for under the requirements of this provision, Pakistan's drug manufacturers and importers need to invest in new technologies, carry out major process changes, and even reorganize the whole supply chain.
Jawed Akhai, another former minister of PPMA, held the same opinion, claiming that the realization of serialization and barcode system would take more than six months and only companies that have been utilizing these technologies for years are likely to meet the requirements within the deadline.
By Ddu
Read more on
- 10 Triumphant Drug Launches Of The Decade August 26, 2021
- China’s Import and Export Market Report of Rheumatoid Arthritis Drugs August 26, 2021
- China’s Import and Export Market Report of Diabetes Drugs August 25, 2021
- China’s Import and Export Market Report of Contraceptive Drugs August 24, 2021
- What ingredients are in vaccines? December 17, 2019
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



