Implanted continuous glucose sensor proven safe
February 28, 2018
Source: globalbiotechinsights
 1,103
1,103
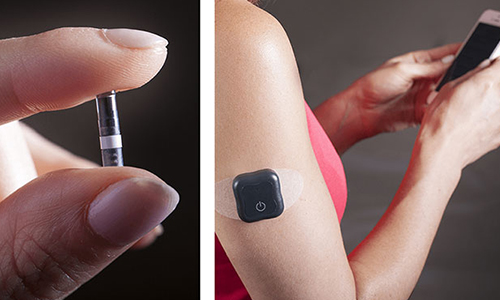
Senseonics Holdings Inc has announced that people with diabetes using the Eversense® brand Continuous Glucose Monitoring Systems, in EMEA markets now have the option to invite others to remotely view their real-time glucose readings and alerts from anywhere.
The updated app with remote monitoring allows up to 5 family members or friends to use Eversense® NOW, a remote monitoring application that automatically updates and displays glucose data sent from the Eversense system every 5 minutes to a mobile device. The Eversense® NOW user can remotely monitor the Eversense® user's CGM data in real time and be alerted of high or low glucose events, including predicted low/high glucose and glucose rate of change status. For more information on glucose monitoring see the IDTechEx report technologies for diabetes management.
"We are thrilled to bring this new product to people with diabetes, their families, and friends. The extra peace of mind and sense of community that comes when someone else can see your real-time glucose data is an important part of a comprehensive solution we want to offer to our customers," said Tim Goodnow, CEO and President of Senseonics. Results of the PRECISE II study showed the implanted continuous glucose monitoring system from Eversense to be safe and highly accurate over the 90-day sensor life in patients with type 1 or type 2 diabetes. More than 93% of CGM glucose values were within an acceptable range of reference values, according to the PRECISE II data reported in Diabetes Technology & Therapeutics, a peer-reviewed journal from Mary Ann Liebert Inc, publishers.
Lynne Kelley, MD, Senseonics, Inc. (Germantown, MD), and a team of clinical researchers from across the United States conducted the prospective, multicenter PRECISE II study. They describe the study design and their findings in the article entitled "A Prospective Multicenter Evaluation of the Accuracy of a Novel Implanted Continuous Glucose Sensor: PRECISE II."
The primary endpoint of the study was the mean absolute relative difference between Eversense glucose values and reference measurements (from 40-400 mg/dL) over the 90-day post-insertion period. The researchers showed that clinicians with limited to no surgical experience could safely insert and remove the CGM sensor after appropriate training. Only one serious adverse event was reported related to device use or sensor insertion/removal.
"Continuous glucose monitoring is becoming standard of care especially for insulin-requiring patients with diabetes. Eversense, if approved by the FDA, will become the first implantable CGM system for use lasting at least 3 months," says DTT Editor-in-Chief Satish Garg, MD, Professor of Medicine and Pediatrics at the University of Colorado Denver (Aurora).
Read more on
- 【EXPERT Q&A】What are the regulations and requirements for exporting medical devices to the European Union? September 5, 2023
- 【EXPERT Q&A】What is the procedure for registering medical devices for the Russian market? August 22, 2023
- Things to Know before Buying Newborn Baby Incubators March 31, 2022
- Bayer’s Kerendia approved in the US to slow CKD in type 2 diabetes patients August 26, 2021
- Autoantibody order, timing predict genetically at-risk children most likely to get type 1 diabetes October 30, 2020
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



