Latest Updates on GMP in Thailand
November 2, 2017
Source: Ddu
 1,382
1,382

Thailand may be one of the countries that pose the biggest challenge for companies who would like to register their pharmaceutical products in Southeast Asia since they require more than your standard documents.
According to the regulations of Thailand’s Ministry of Health, every foreign pharmaceutical manufacturer must have a GMP before applying for product registration. New regulations regarding GMPs were issued by Thailand’s Ministry of Health last year. In June of this year, the newest regulations of GMP in Thailand were finally issued to the public.
Though Thailand is a member of PIC/S, their requirements for GMPs of non-PIC/S countries differ from those of other PIC/S countries. This can be divided into the following categories according to specific countries and regions:
- PIC/S countries
- Non-PIC/S countries with GMPs issued by PIC/S countries
- Non-PIC/S countries without GMPs issued by PIC/S countries
Ddu herewith explains the specific requirements in detail to you.
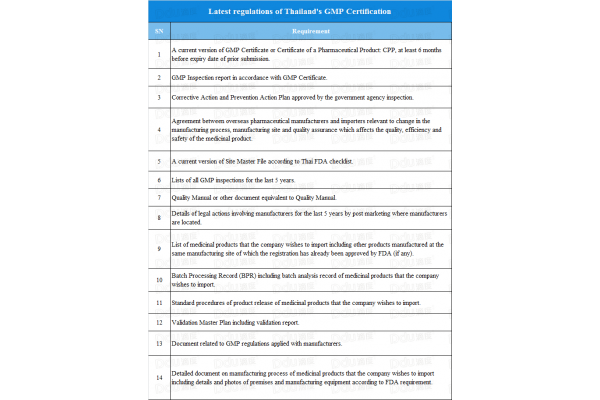
Taking into consideration the locations of companies and the requirements mentioned in the form above, the following conclusions are of interest:
- PIC/S countries are required to meet the requirements of no. 1 to no. 4
- Non-PIC/S countries with GMPs issued by PIC/S countries are required to meet the requirements of no. 1 to no. 5
- Non-PIC/S countries without GMPs issued by PIC/S countries, are required to meet the all requirements mentioned above.
The document makes it clear that there are huge differences in the requirements for PIC/S countries and non-PIC/S countries. However, don’t let this discourage you as GMP standards and related documents are gradually being perfected and Thailand may grant approvals.
As the leading global pharmaceutical & medical device B2B online platform, Ddu will continue bring you the latest updates on Thailand’s Ministry of Health and other news from Asia.
By DduRead more on
- 10 Triumphant Drug Launches Of The Decade August 26, 2021
- China’s Import and Export Market Report of Rheumatoid Arthritis Drugs August 26, 2021
- China’s Import and Export Market Report of Diabetes Drugs August 25, 2021
- China’s Import and Export Market Report of Contraceptive Drugs August 24, 2021
- What ingredients are in vaccines? December 17, 2019
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



