Boao Lecheng CGT project reaches 20 items! The first autologous NK therapy for eight types of cancer in China has been approved, costing 49,800 yuan per treatment
January 31, 2026
Source: https://mp.weixin.qq.com/s/pjUOs4554LmRv1uJdHWQow
 32
32

Recently, the nation's first autologous NK cell adjuvant therapy technology covering eight types of tumor indications was officially approved for clinical application at West China Lecheng Hospital.
The cost of a single treatment session is 49,800 yuan, with a total of 6 sessions per course, and the total cost for the entire course is approximately 298,800 yuan. This treatment covers lung cancer, breast cancer, stomach cancer, pancreatic cancer, ovarian cancer, liver cancer, prostate cancer, and colorectal cancer.
According to a January 28 report from the Boao Lecheng International Medical Tourism Pilot Zone Administration, with the recent approval of four new cell and gene therapy (CGT) projects, Lecheng has now achieved a total of 20 CGT projects that have been translated into clinical applications.
Recent additions include:
Shulan (Boao) Hospital: Autologous NK Cell Therapy Project
Ciming Boao International Hospital: Umbilical cord mesenchymal stem cell therapy for type 2 diabetes (for patients whose blood sugar is still poorly controlled after using at least three hypoglycemic drugs in combination) .
Ruijin Hainan Hospital: KSD-101 Dendritic Cell (DC) Drug (for the treatment of EBV-related hematologic malignancies, from Hengsai Biotechnology)
Boao Super Hospital: German DC Immunotherapy Technology
Previously, 15 CGT projects had been launched in Boao Lecheng.
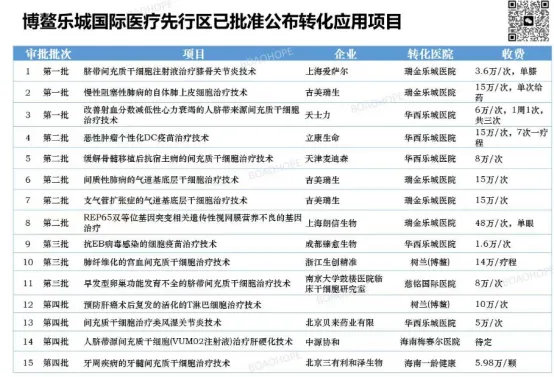
Currently, the Lecheng Administration has issued a notice soliciting applications for the third batch of biomedical new technology transformation and application projects, with the application deadline being March 9, 2026.
By editorRead more on
- Strategic Collaboration with AstraZeneca, Potential Transaction Value Up to $18.5 Billion January 31, 2026
- Farsight Medical Announces First Patient Dosed in Phase II Clinical Trial of RN-0001, a World-First Therapy for Acute Pancreatitis January 31, 2026
- Yangtze River Pharmaceutical Group is ramping up its investment in the 10 billion yuan contrast agent market, aiming to challenge Hengrui January 31, 2026
- Simcere Pharmaceutical’s TL1A/IL-23p19 bispecific antibody goes global, partnering with Boehringer Ingelheim to expand the global inflammatory bowel disease market. January 31, 2026
- Within 29 days of its listing, Insilicon has secured four collaborations. January 31, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



