The world’s first clinically approved “autologous regenerated islets” for the treatment of type 2 diabetes! Ruipuchen Chuang opens a new dual-indication autologous regeneration track.
January 30, 2026
Source: https://mp.weixin.qq.com/s/EtUZYv7EphTZ59KW-Jh9Lw
 33
33

On January 29, the Investigational New Drug (IND) application for RGB-5088 pancreatic islet cell injection, independently developed by Hangzhou Ruipuchenchuang Technology Co., Ltd., for the treatment of type 2 diabetes was officially approved by the Center for Drug Evaluation (CDE) of the National Medical Products Administration.
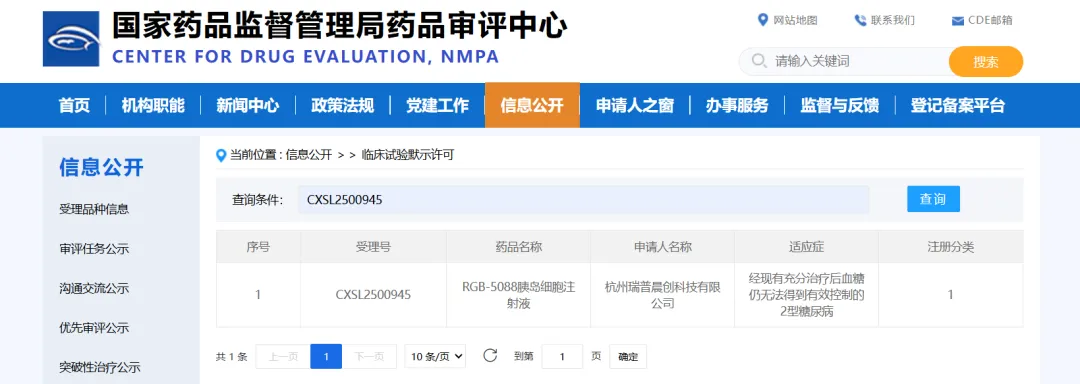
This is the world's first product to utilize autologous regenerated islet therapy to treat type 2 diabetes . "Autologous regenerated islet therapy" refers to the process of creating islet cells from the patient's own cells to replace the patient's dysfunctional islet cells, thereby achieving long-term stable control of blood sugar levels. The islet cells created by this product not only closely resemble adult islet cells in structure and function but also possess high glucose sensitivity, allowing them to regulate insulin secretion based on changes in blood glucose concentration.
It is worth noting that RGB-5088 islet cell injection has previously been approved for clinical trials in type 1 diabetes.
Therefore, Ruipuchenchuang's RGB-5088 pancreatic islet cell injection has entered clinical trials for both type 1 and type 2 diabetes.
This is the world's first autologous regenerated islet therapy product covering both type 1 and type 2 diabetes.
For a long time, the treatment of type 2 diabetes has relied heavily on oral hypoglycemic agents and insulin injections, focusing more on blood sugar control than on curing the disease, requiring patients to maintain long-term medication. However, RGB-5088, through autologous regenerated islet cell transplantation, replaces the body's dysfunctional islet cells, potentially overcoming the limitations of traditional treatments and offering patients a breakthrough treatment option for functional cure.
According to statistics from the International Diabetes Federation, the number of people with diabetes worldwide is approaching 600 million, of which approximately 90% have type 2 diabetes. In my country, the total number of people with diabetes is nearly 150 million, also predominantly type 2 diabetes. The simultaneous advancement of RGB-5088 for dual indications is expected to benefit the vast majority of diabetes patients worldwide in the future, unlocking broad clinical and social value.
By editorRead more on
- Initial Clinical Exploration of D-0502 in Combination with ADC Therapies Officially Commenced. January 30, 2026
- Pfizer’s blockbuster drug receives notice of approval! January 30, 2026
- Aminophylline tablets pass generic drug consistency evaluation January 30, 2026
- Propafenone Hydrochloride Injection Receives Drug Production Registration Certificate January 30, 2026
- Hengrui’s innovative drug camrelizumab in combination with apatinib mesylate and TACE for the treatment of liver cancer has been accepted for marketing authorization application January 30, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



