The Phase 1 clinical trial showed amazing results, causing the stock price to surge by 166%.
January 23, 2026
Source: drugdu
 31
31
Corvus Pharmaceuticals recently released updated Phase 1 clinical data on the ITK inhibitor Soquelitinib for the treatment of atopic dermatitis.
Corvus Pharmaceuticals' stock price surged 166% that day , reaching a market capitalization of $1.6 billion.
Soquelitinib blocks the TCR pathway, increasing Th1 while downregulating Th2 and Th17, the latter two playing important roles in autoimmune diseases.
Soquelitinib is a selective ITK inhibitor and the world's first ITK inhibitor, with potential indications being explored for PTCL, AD, and other indications.
Atopic dermatitis still presents significant unmet clinical needs, with 50% of patients failing biologic therapy.
Soquelitinib has updated the data from Phase 1 cohort 4 of its clinical trial, with the clinical protocol design as follows.
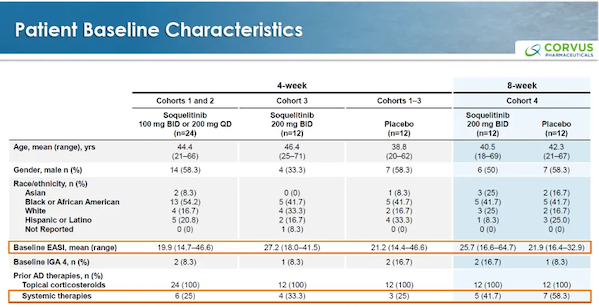
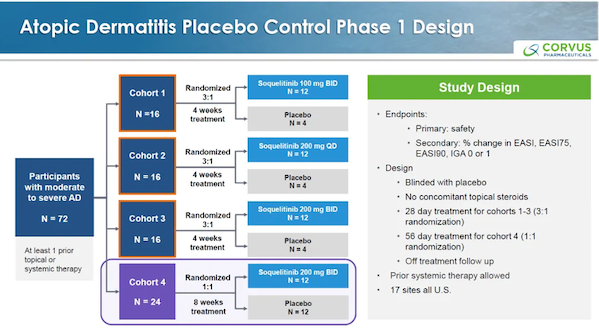 The patient's baseline information is as follows.
The patient's baseline information is as follows.
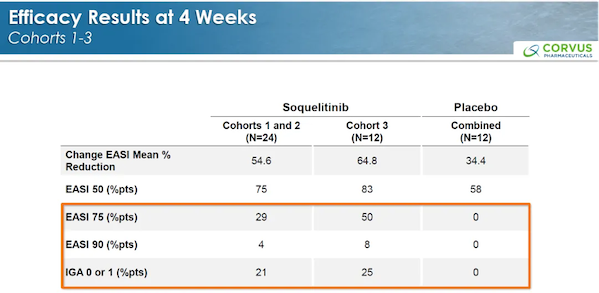
 The previously released efficacy data for cohorts 1-3 after 4 weeks of treatment are as follows.
The previously released efficacy data for cohorts 1-3 after 4 weeks of treatment are as follows.
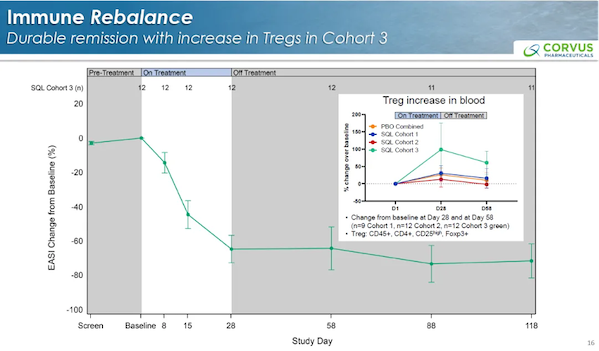
 In cohort 3, treatment was discontinued after 4 weeks, and the therapeutic effect persisted, which was associated with a sustained increase in Tregs.
In cohort 3, treatment was discontinued after 4 weeks, and the therapeutic effect persisted, which was associated with a sustained increase in Tregs.
 Soquelitinib still shows excellent efficacy in patients who have received other systemic therapies, including IL-4R antibodies, JAK inhibitors, hormones, etc.
Soquelitinib still shows excellent efficacy in patients who have received other systemic therapies, including IL-4R antibodies, JAK inhibitors, hormones, etc.
Conclusion
The updated cohort 4 data demonstrates the significant potential of soquelitinib, with phase II clinical trials for atopic dermatitis, asthma, and hidradenitis suppurativa to be initiated soon. ITK inhibitors not only boast impressive efficacy data but also exhibit durable efficacy and low relapse rates at the mechanism of action level, potentially revolutionizing the treatment landscape for these indications.
https://news.yaozh.com/archive/46971.html
By editorRead more on
- RiboBio’s ApoC3-Targeting siRNA Drug RBD5044 Granted Implicit Approval for Phase II Clinical Trials in China January 23, 2026
- A new generation of ophthalmic dual antibodies challenges Roche’s dominant position. January 23, 2026
- Leading CRO company sees net profit surge 1468% January 23, 2026
- Robotic diagnosis and treatment is reaching a turning point towards widespread adoption. January 23, 2026
- Wanshuan® (aniline profen injection) is Yiling Wanzhou’s first approved patented new chemical drug. January 23, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



