New Advances in Hand Eczema Treatment
August 26, 2025
Source: drugdu
 134
134
LEO Pharma recently announced that the FDA has approved ANZUPGO (delgocitinib) Cream (20 mg/g) for the topical treatment of moderate-to-severe chronic hand eczema (CHE) in adults. Delgocitinib is a pan-JAK inhibitor that simultaneously inhibits JAK1, JAK2, JAK3, and TYK2, suppressing the signaling of proinflammatory cytokines (such as IL-4, IL-13, and IL-23) and alleviating skin inflammation and itching.
Degatinib cream is the world's first topical JAK inhibitor specifically used to treat moderate to severe chronic hand eczema and is expected to become a new treatment option for CHE patients.
Principles and R&D progress
Hand eczema is the most common hand skin disease, presenting as a variety of lesions including erythema, papules, blisters, scaling, hyperkeratosis, and fissures. Most patients with hand eczema develop the condition into a chronic condition. Chronic hand eczema (CHE) refers to hand eczema that persists for more than three months or recurs two or more times within a year, often accompanied by secondary skin infections, persistent pain, and intense itching. It has a high morbidity rate, a heavy disease burden, and severely impacts daily life.
Studies have shown that among patients who developed hand eczema in the past year, chronic hand eczema accounted for approximately 63.8%, with most cases being moderate to severe. The severity of hand eczema is highly correlated with patients' quality of life. Over half of patients with hand eczema are caused by occupational exposure, with a high prevalence among occupationally exposed groups such as medical personnel (frequent handwashing), hairdressers (exposure to hair dyes), and workers (exposure to organic solvents).
The etiology of hand eczema is complex and can be divided into exogenous and endogenous factors. Exogenous factors are mostly contact factors and mechanical damage, including: ① contact allergens, such as metal products and food proteins; ② contact irritants, such as strong irritants such as acids, alkalis, and organic solvents, or weak irritants such as water, soap, detergents, motor oil, and printed materials; and ③ mechanical damage, such as trauma, scratching, and long-term friction. Endogenous factors mainly include genetic factors, atopic constitution, mental state, hormone levels, immune status, and changes in trace elements. Atopy plays a dominant role among endogenous causes, and those with a history of atopic dermatitis have a significantly higher risk of developing hand eczema than those without a history of AD. In patients with atopic constitution, the Janus kinase (JAK) signaling pathway and IL-31 released by Th2 play an important role in the itch symptom.
JAK inhibitors target the JAK-STAT (signal transducer and activator of transcription) pathway, which plays a key role in the pathogenesis of many immune-mediated diseases, such as AD. Although the efficacy of JAK inhibitors for AD in HE is not fully established, inhibition of the Th2 pathway may be beneficial in allergic contact dermatitis, as many allergens can trigger a Th2-biased immune response.
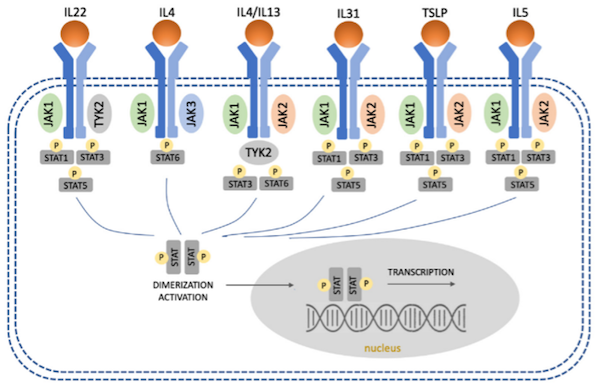 Figure 1 JAK-STAT signaling pathway
Figure 1 JAK-STAT signaling pathway
Image source: Literature
Currently, there are two JAK inhibitor drugs on the market for the treatment of hand eczema, namely Incyte's ruxolitinib and Japan Tobacco's dextrotinib. Ruxolitinib has been approved for marketing in China. In addition, two JAK inhibitors for the treatment of hand eczema have entered the clinical stage worldwide.
Developed by Incyte, ruxolitinib was initially used to treat myeloproliferative diseases such as myelofibrosis. It was approved by the FDA in November 2011 under the trade name Jakavi and subsequently approved for the treatment of indications such as graft-versus-host disease. In September 2021, the FDA approved ruxolitinib cream under the trade name Opzelura for the short-term and discontinuous long-term treatment of mild to moderate atopic dermatitis (AD) in patients aged 12 years and older. It was subsequently approved for the treatment of non-segmental vitiligo. Thanks to its broad indication coverage and excellent clinical data, ruxolitinib is expected to achieve global sales of US$4.728 billion in 2024, maintaining steady growth.
Ruxolitinib phosphate tablets were approved for marketing in China under the priority review process in March 2017, under the brand name Jervoy. They were included in the national medical insurance system in 2019. Currently, approved indications for ruxolitinib phosphate tablets in China include graft-versus-host disease and myelofibrosis. The rates of ruxolitinib phosphate tablets in China were 29.1% vs. 6.9% and 49.5% vs. 18.2%, respectively. These results were statistically significant (p<0.001).
Degotinib was developed by Japan Tobacco Inc. (JT), and LEO Pharma has been granted exclusive rights to develop and commercialize it worldwide for dermatological indications, excluding Japan. Degotinib is a pan-JAK inhibitor that simultaneously inhibits JAK1/2/3 and TYK2 targets. By blocking the JAK-STAT pathway, it suppresses the signaling of proinflammatory cytokines (such as IL-4, IL-13, and IL-23), thereby alleviating skin inflammation and pruritus. Currently, degotinib has been approved in the United States, the European Union, the United Kingdom, Switzerland, the United Arab Emirates, and Macau, China, for the treatment of adult patients with moderate to severe chronic hand eczema (CHE) who have had an inadequate response to or are not suitable for corticosteroids.
Preliminary results from the Phase III DELTA 2 clinical trial of delgocitinib showed that at week 16 and in additional analyses, the proportion of patients using delgocitinib cream who achieved the primary endpoint (IGA-CHE score of 0 or 1 and improvement of ≥2 points from baseline at week 16) and the key secondary endpoint (HECSI-75 index improvement of ≥75%) was significantly increased compared with patients using the cream vehicle.
In China, the first patient was enrolled and dosed in a Phase III clinical trial of degotinib cream for moderate to severe chronic hand eczema in adults at the Southern Medical University Dermatology Hospital in September 2023. On February 27, 2025, LEO Pharma announced that the Phase III DELTA clinical trial of degotinib cream for moderate to severe chronic hand eczema (CHE) in adults and adolescents (12 years and older) who had an inadequate response to or were not suitable for topical corticosteroids met its primary endpoint.
Unmet clinical needs
Epidemiological surveys and studies have shown that hand eczema is a common chronic skin disease in clinical practice, with a global incidence of approximately 5.5-8.8 cases per 1,000 person-years. The incidence rate in women is higher than that in men. The one-year prevalence is approximately 5.2%-10%, and the lifetime prevalence can reach 14.5%. About one-third of patients have moderate to severe conditions and have a history of atopic dermatitis (AD).
The clinical manifestations of hand eczema are complex and diverse. It can be classified according to the length of the course of the disease into acute hand eczema (course ≤ 3 months or ≤ 1 attack within 1 year) and chronic hand eczema (course > 3 months or ≥ 2 recurrences per year). The proportion of chronic hand eczema is about 2/3.
The pathogenesis of CHE has not been fully elucidated, and it is difficult to improve patients' symptoms through a single approach. It cannot be cured at present. The current prevention and treatment points are mainly to combine multiple factors such as the cause, course of disease, severity, characteristics of skin lesions, etc., to comprehensively formulate individualized plans, actively find the cause, clarify the diagnosis, control complications, improve symptoms as soon as possible, and delay and alleviate recurrence.
Currently, the drug treatment regimen for CHE is generally multimodal treatment. Topical treatment is the standard treatment for patients with mild CHE, mainly including emollients, topical corticosteroids and topical calcineurin inhibitors, but up to 65% of cases cannot be relieved after treatment; moderate to severe cases are usually resistant to topical treatment and require systemic treatment. Treatment options include physical therapy such as ultraviolet light therapy and systemic drug therapy, including systemic use of steroids, retinoids and immunosuppressants.
Current CHE treatments still fall short of addressing the full range of treatment needs for CHE patients. Existing therapies, such as topical steroids and calcineurin inhibitors, carry risks of skin atrophy and drug resistance with long-term use, and their efficacy is often insufficient. Systemic immunosuppressants, while effective, are associated with significant side effects and require close monitoring. Many patients continue to face challenges such as recurrent illness, severe itching, and poor quality of life, and new, more effective, safe, and accessible treatment options are urgently needed.
Conclusion
As the world's first topical pan-JAK inhibitor, degotinib cream fills a gap in targeted treatment for moderate to severe chronic hand eczema (CHE), offering a new option for patients who have a poor response to or are unsuitable for traditional steroid therapy. By simultaneously inhibiting JAK1/2/3 and TYK2 targets, it effectively blocks the signaling pathways of pro-inflammatory factors such as IL-4 and IL-13, significantly alleviating skin inflammation and pruritus. The Phase III DELTA clinical trial achieved its primary endpoint, confirming its superior efficacy and safety. Compared to systemic JAK inhibitors, degotinib cream offers advantages such as local administration, low systemic exposure, and ease of use, making it more suitable for long-term disease management. As its approval process in China progresses, it is expected to significantly improve the quality of life of CHE patients, promote the alignment of targeted eczema treatment in China with international standards, and provide evidence-based support for the expansion of its use into more indications, such as atopic dermatitis.
https://news.yaozh.com/archive/45951.html
By editorRead more on
- Phase III clinical trial of vetcotozumab completes patient enrollment February 9, 2026
- The first long-acting coagulation factor VIII, has officially entered the Chinese mainland market. February 9, 2026
- 17.9 billion yuan! A top-selling topical medication emerges. February 9, 2026
- Turnaround! Generic drug giant successfully “revived” February 9, 2026
- Novartis’s first-ever autoimmune drug has been submitted for marketing approval in China. February 9, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



