A new hotspot in the innovative drug market
July 25, 2025
Source: drugdu
 227
227

After a long period of financial difficulties, French biotech company Abivax announced positive top-line results from two Phase III studies (ABTECT-1 and ABTECT-2) of its microRNA (miRNA) drug Obefazimod (ABX464) for the treatment of ulcerative colitis (UC).
Obefazimod is an oral, first-in-class enhancer of miR-124 (an anti-inflammatory miRNA) developed by Abivax.
ABTECT-1 (Study 105) and ABTECT-2 (Study 106) included a total of 1,275 patients worldwide. The enrollment population was balanced, and 47.3% of the subjects had insufficient responses to previous advanced therapies (biological agents/JAK inhibitors, etc.), including the largest JAK inhibitor failure population in Phase III UC trials to date. The primary endpoint was the clinical remission rate based on the modified Mayo score after 8 weeks of treatment.
The results showed that in the pooled analysis of the two trials, the placebo-corrected clinical remission rate of 50 mg of obefazimod once daily reached 16.4% (p<0.0001). At the same time, the 50 mg dose group achieved all key secondary endpoints, which was statistically significant and clinically meaningful.
In the ABTECT-1 study, the remission rates in the 25 mg and 50 mg groups were significantly better than those in the placebo group, at 23.8% and 21.7%, respectively, compared to 2.5% in the placebo group.
In the ABTECT-2 study, the clinical remission rate in the 50 mg group was significantly better than that in the placebo group, at 19.8% and 6.3%, respectively.
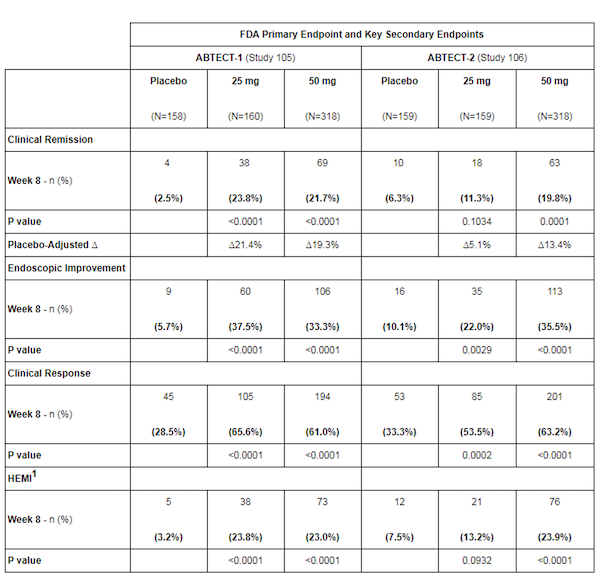 Figure 1 Results of ABTECT-1 and ABTECT-2 studies
Figure 1 Results of ABTECT-1 and ABTECT-2 studies
Image source: Abivax official website
In terms of safety, Obefazimod was generally well tolerated, and the safety data were consistent with previous studies, with no new risks found. The incidence of headaches was lower than in earlier trials and was transient, mostly disappearing within a few days of the initial treatment.
Currently, the ABTECT project is still ongoing, with 678 of the 1,275 induction trial patients entering the ABTECT maintenance trial (ABX464-107), and key data are expected to be read out in the second quarter of 2026.
After the data was released, Abivax's stock price soared 586%, successfully attracting the market's keen attention to miRNA drugs. Abivax took advantage of the situation to launch a financing plan with a total amount of approximately US$400 million (approximately EUR 340 million) for the development of indications such as ulcerative colitis and Crohn's disease, as well as working capital and general corporate purposes.
MicroRNA (miRNA) is a type of endogenous non-coding small RNA with a length of 20 to 24 nucleotides. It is widely present in eukaryotes and precisely regulates the expression of target genes by binding to specific mRNA through sequence complementarity. It plays an important role in cell differentiation, development, immune response and disease occurrence. The 2024 Nobel Prize in Physiology or Medicine was awarded to American scientists Victor Ambros and Gary Ruvkun in recognition of their outstanding contribution to "the discovery of miRNA and its role in post-transcriptional gene regulation", suggesting the huge application potential of miRNA drugs.
Titer data shows that no miRNA drugs have been approved for marketing in the world, and there are fewer companies investing in them than in siRNA and ASO drugs, so it is still a blue ocean field. Among them, Causeway's CWT-001 and InteRNA's INT-1B3 are making rapid progress, and Apic Bio and UniQure also have a number of miRNA drug pipelines.
Chinese companies, most pipelines in the field of miRNA therapy are still in the preclinical stage, and companies with layout include Yunhai Tetrahedron, Jimu Bio, New Osion Pharmaceuticals, and Jiankai Technology.
https://news.yaozh.com/archive/45826.html
By editorRead more on
- Phase III clinical trial of vetcotozumab completes patient enrollment February 9, 2026
- The first long-acting coagulation factor VIII, has officially entered the Chinese mainland market. February 9, 2026
- 17.9 billion yuan! A top-selling topical medication emerges. February 9, 2026
- Turnaround! Generic drug giant successfully “revived” February 9, 2026
- Novartis’s first-ever autoimmune drug has been submitted for marketing approval in China. February 9, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



