Connoya sprints to become the “King of Chinese Self-immunity”
June 23, 2025
Source: drugdu
 193
193
Recently, NEJM published the breakthrough data of Conoya's BCMA/CD3 dual antibody CM336 in the treatment of refractory autoimmune hemolytic anemia (AIHA), with significant and lasting efficacy. This is not only a strong proof of its innovative strength, but also forms a synergy with the rapid commercialization of its trump card CM310, strongly supporting its sprint to become the king of autoimmune in China.
BCMA/CD3 dual antibody autoimmune new blue ocean
Bispecific antibody technology with T cell redirection as the core mechanism has made significant breakthroughs in the field of hematological tumors. Among them, BCMA/CD3 bispecific antibodies effectively guide cytotoxic T lymphocytes (CTL) to target and kill tumor cells through their unique dual antigen binding ability. Globally, two drugs, Teclistamab from Johnson & Johnson and Elranatamab from Pfizer, have been approved for relapsed and refractory multiple myeloma (RRMM). The former will land in the Chinese market in June 2024. Johnson & Johnson expects its peak sales to exceed US$5 billion, which fully confirms the huge value of this target in the field of hematological tumors.
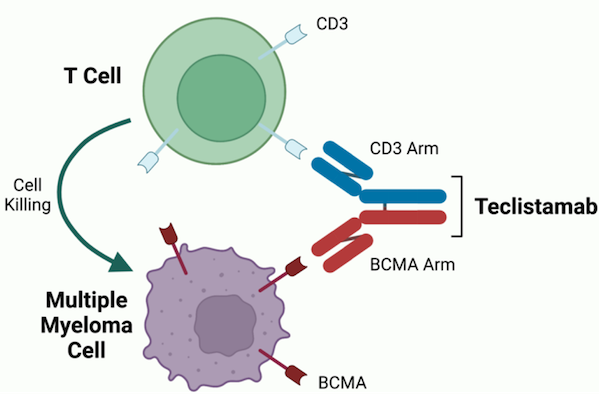 Figure Teclistamab mechanism of actio
Figure Teclistamab mechanism of actio
When the therapeutic potential transcends disease boundaries, the vast market of autoimmune diseases is becoming the next strategic blue ocean for BCMA/CD3 bispecific antibodies. B cell dysfunction is the core pathogenesis of many autoimmune diseases. CD3, as a key subunit of the T cell receptor complex, has been shown to be closely related to diseases such as SLE. BCMA, as an important surface marker of plasma cells, naturally becomes an ideal target for clearing the source of pathogenic autoantibodies.
In September 2024, the breakthrough results of Johnson & Johnson's Teclistamab in the treatment of a severe SLE patient provided exciting clinical validation for this theory. B cells and plasma cells in patients treated with Teclistamab were rapidly cleared, accompanied by a significant decrease in proteinuria, achieving complete remission independent of medication. The drug also showed significant efficacy in patients with other refractory autoimmune diseases, preliminarily revealing the potential of BCMA/CD3 dual antibodies to reshape the landscape of autoimmune treatment.
More importantly, international capital's favor for domestic BCMA/CD3 bispecific antibodies further demonstrates the huge potential and commercial value of autoimmune diseases.
In September 2024, EpiMed Bio granted the global rights to its BCMA/CD3 bispecific antibody EMB-06 to Vignette Bio. The down payment and equity consideration for the transaction reached US$60 million, and potential milestone payments were as high as US$575 million.
In November 2024, Weilizhibo, with its innovative CD19/BCMA/CD3 triple antibody LBL-051, co-founded Oblenio Bio with Aditum Bio and obtained financial support to accelerate clinical advancement;
In November 2024, Conoya also reached an agreement with Platina Medicines to grant the latter overseas rights to CM336, and received an initial payment of US$16 million and milestone payments of up to US$610 million.
These high-value cross-border collaborations all demonstrate that BCMA/CD3 bispecific antibodies are rapidly expanding into the broader "new blue ocean" of autoimmune disease.
The next generation of autoimmune products
Conoya is relying on the rapid commercialization of CM310 and the accelerated advancement of its differentiated pipeline to build the next generation of autoimmune "core products".
Among them, its autoimmune "ace" product CM310 (Sipuqibaimab) has achieved a major breakthrough in domestic production and demonstrated the potential for continued expansion. As the first domestically produced and the second approved IL-4Rα monoclonal antibody in the world, CM310 is precisely targeted at the global autoimmune "new king" Dupilumab (Dupixent). Dupilumab's sales of up to US$14.179 billion in 2024 and its successful strategy of "one drug for multiple diseases" strongly confirm the huge market space and growth potential of the IL-4Rα target.
CM310 is rapidly replicating this successful path. After being first approved for adult atopic dermatitis (AD) in September 2024, it has also been approved for chronic sinusitis with nasal polyps (CRSwNP) and allergic rhinitis indications in just half a year. Its adolescent AD and asthma indications are already in Phase III clinical trials. The continued expansion of indications in the future will provide a solid guarantee for the long-term market expansion of the product, making it the core growth driver supporting Conoya's autoimmune map.
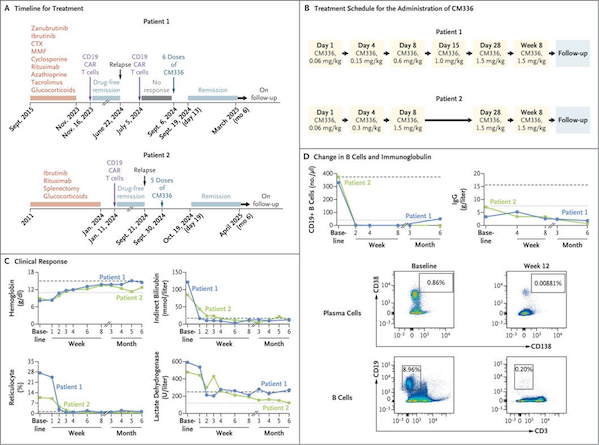 Figure CM336 Clinical Data
Figure CM336 Clinical Data
In addition, as a BCMA/CD3 bispecific antibody developed by Conoya, CM336 bridges T cells with target cells expressing BCMA through its unique mechanism, activating T cell-mediated specific killing. In June 2025, its breakthrough case for the treatment of refractory autoimmune hemolytic anemia (AIHA) was published in the New England Journal of Medicine (NEJM). Studies have shown that two patients who relapsed after multiple lines of treatment (including CD19 CAR-T) quickly achieved remission and maintained efficacy for more than 6 months after CM336 rescue treatment, with good safety. Its overseas partner Ouro Medicines plans to launch relevant clinical research in the second half of 2025, and the internationalization process will be accelerated simultaneously.
In addition to core products and breakthrough therapies, Conoya's multiple early pipelines in the autoimmune field are also worth looking forward to. TSLP monoclonal antibody CM326 started a Phase II clinical study for the treatment of CRSWNP in May 2024, and its clinical progress is the first echelon of domestic products; TSLPxIL-13 dual antibody CM512 is currently in Phase I clinical trials for AD indications, exploring the synergistic effect of dual targets; CM313 is the first domestically approved CD38 monoclonal antibody, and its Phase I data in immune thrombocytopenia (ITP) have shown good efficacy and safety, and is currently in the Phase II clinical study stage.
Leading the new trend of NewCo going overseas
In the journey of innovative pharmaceutical companies seeking to break through internationalization, the NewCo model is becoming a key breakthrough path. According to incomplete statistics, at least six local companies have adopted this strategy to go overseas. The NewCo model not only opens up a way for Chinese biotech to obtain cash flow outside the strict introduction standards of MNCs and support research and development, but also helps to disperse the risks of local pharmaceutical companies. Faced with China's demand for funds, technology, and markets, as well as fierce external competition and financing pressure, NewCo has opened up a new path for the internationalization of China's innovative drugs.
In this wave of exploration, Conoya has taken the lead with its outstanding achievements. In just over a year, Conoya has set sail for the fourth time in the NewCo model, with a total transaction volume approaching US$1.7 billion.
Specifically, in July 2024, the rights outside Greater China for the bispecific new drugs CM512 and CM536 were granted to Belenos, which was co-founded with OrbiMed Capital; in November of the same year, the BCMA/CD3 bispecific antibody CM336 was successfully joined hands with Platina; in January 2025, the potential best-in-class monoclonal antibody CM313 targeting CD38 was licensed to Timberlyne, which was co-founded with Bain and others, and became its largest shareholder; almost at the same time, it joined hands with InnoCare Pharma to grant the non-tumor global and non-Asian tumor rights of the CD20×CD3 bispecific antibody CM355 (ICP-B02) to Prolium.
These four intensive and successful overseas practices clearly outline Conoya's strategic layout.
First, it is to seize the high ground with the cutting-edge innovative product matrix. Among the four products exported by Conoya, three are bispecific antibodies with high technical barriers and broad prospects, which strongly occupy half of the Chinese bispecific antibody NewCo overseas products since 2024. Another CD38 monoclonal antibody CM313 breaks through the traditional hematological tumor framework and proactively deploys in the autoimmune field, showing significant potential to become "Best-in-class";
Secondly, it is the company that dares to and is good at realizing value globalization at an early stage. CM536 is in the preclinical stage, CM512 is in the clinical application stage, CM336 and CM355 are in Phase I/II, and CM313 is in Phase II. The partners are willing to bet on Conoya at such an early stage, which is not only a high recognition of its "hard power" in scientific research and innovation, but also demonstrates the company's forward-looking vision in its internationalization strategy;
Finally, through this NewCo model of "deep bundling and leading process", Conoya ensures its influence on the global destiny of its innovation pipeline, while optimizing resource allocation and internationalization efficiency.
Conclusion
With "hard-core" products in hand and accelerated pipeline layout, Conoya is unstoppable in its bid to become the "King of Self-Immunity in China".
https://news.yaozh.com/archive/45651.html
By editorRead more on
- Phase III clinical trial of vetcotozumab completes patient enrollment February 9, 2026
- The first long-acting coagulation factor VIII, has officially entered the Chinese mainland market. February 9, 2026
- 17.9 billion yuan! A top-selling topical medication emerges. February 9, 2026
- Turnaround! Generic drug giant successfully “revived” February 9, 2026
- Novartis’s first-ever autoimmune drug has been submitted for marketing approval in China. February 9, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



