Innovent Biologics enters a new era of dual-wheel drive and global innovation and development
January 22, 2025
Source: drugdu
 410
410
 January 17, 2025, San Francisco, USA and Suzhou, China - Innovent Biologics (HKEX: 01801), a biopharmaceutical company dedicated to the research, development, production and sale of innovative drugs in major disease areas such as oncology, autoimmunity, metabolism, ophthalmology, etc., was invited to attend the 43rd Annual JPMorgan Healthcare Conference (JPM). Dr. Yu Dechao, founder, chairman and CEO of the group, delivered a keynote speech on site to share the company's latest business progress and prospects.
January 17, 2025, San Francisco, USA and Suzhou, China - Innovent Biologics (HKEX: 01801), a biopharmaceutical company dedicated to the research, development, production and sale of innovative drugs in major disease areas such as oncology, autoimmunity, metabolism, ophthalmology, etc., was invited to attend the 43rd Annual JPMorgan Healthcare Conference (JPM). Dr. Yu Dechao, founder, chairman and CEO of the group, delivered a keynote speech on site to share the company's latest business progress and prospects.
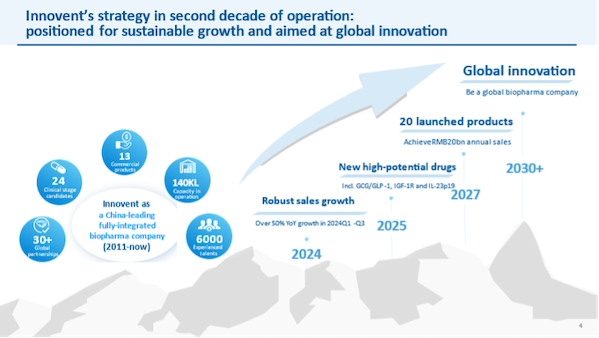
In its 13 years of establishment, Innovent Biologics has grown rapidly into a leading biopharmaceutical company in China. Under the guidance of the "sustainable development and global innovation" strategy, it has continuously achieved excellent results. At the beginning of the new year, the company has made important progress: two lung cancer targeted drugs, Dabolu® and Aoyixin®, were approved in succession, and the number of commercial products increased to 14; Xinbilu® was included in the medical insurance implementation, becoming the first and only Chinese original PCSK9 inhibitor included in the medical insurance; signed a US$1 billion licensing cooperation with Roche to jointly promote the global development of ADC drugs; further expanded strategic cooperation with Eli Lilly to commercialize the latest blood tumor variety Jepali®; concentrated efforts on research and development, IBI363 was approved by the FDA for fast track review, and IBI343 was approved for breakthrough therapy channel, which comprehensively accelerated the internationalization process.
Looking to the future, Innovent Biologics has a clear strategy and is steadily implemented. 2025 will be an important year for entering a new era of dual-wheel drive and global innovation and development. With business momentum moving forward and innovation striving for breakthroughs, the company is steadily moving towards the vision of "becoming a world-class biopharmaceutical company".
Dual-wheel drive, the path of sustainable development is becoming clearer . After years of
hard work, Innovent Biologics has become a leading brand in the field of tumor treatment in China, and continues to promote the launch of new products and late-stage clinical development. At the same time, as the second growth pole of the company's growth, the launch of multiple blockbuster new drugs in 2025 will unlock more market space for chronic diseases.
Innovent Biologics is more confident in achieving the goal of RMB 20 billion in product revenue in China by 2027. While maintaining the rapid growth of business scale, it will continue to improve its lean operation and profit-oriented operation model towards sustainable development.
To build core competitiveness,
Innovent Biologics' innovative R&D engine - Guoqing Institute has established a comprehensive technology platform, including ScFv engineering, T cell connector (TCE), VHH bispecific antibodies, Topo1i ADC, dual-load ADC, antibody-peptide conjugates (APC) and other innovative technologies, to efficiently and continuously produce innovative molecules, providing a source of power for the company's long-term development.
Among them, Innovent Biologics has combined world-leading antibody engineering and multiple sets of differentiated linker-payload technologies to create a highly competitive innovative TOPO1i ADC technology platform and dual-payload ADC technology platform, and has efficiently produced a new generation of potential best-in-class (BIC) or first-in-class (FIC) ADC drug candidates.
To date, the company has 8 ADC drug candidates entering clinical research, and has accumulated efficacy and safety data from more than 600 patients. Multiple ADC molecules have obtained breakthrough therapy designation (BTD), fully validating the advanced nature of the company's ADC platform technology and its consistent clinical execution capabilities.
The company continues to focus on core high-potential disease areas, and continues to strengthen its leadership in the field of tumors through a rich and powerful product pipeline of "PD-1+precision therapy". Under the new generation of "IO+ADC" strategy, breakthroughs in innovative therapies are expected to bring about changes in cancer treatment. At the same time, the company's comprehensive pipeline covers autoimmunity, cardiovascular and metabolic, ophthalmology, etc. The new generation of innovative drugs will improve the treatment standards for a wide range of disease populations.
Global business opens up future upward space.
Guoqing Institute has been preparing for many years, and the new generation of pipelines has ushered in new opportunities for global development. Innovative pipelines represented by new generation IO and ADC have successively ushered in new global development opportunities. In the future, more innovative ADCs, bispecific (multispecific) antibodies, next-generation autoimmune and CVM pipelines will gradually enter global development.
IBI343 (CLDN18.2 ADC): The Phase III multi-regional clinical study (MRCT) for gastric cancer in China and Japan has been initiated. The Phase I clinical MRCT for pancreatic cancer has read positive efficacy and safety signals in the Chinese population, and has started partial clinical enrollment in the United States. In 2025, after the PoC verification data is read out, it is expected to start international key clinical studies.
IBI363: As the world's first (FIC) PD-1/IL-2α dual-antibody drug, it has read positive Phase I clinical data in hundreds of core tumor types such as IO-resistant lung cancer, melanoma, and IO-unresponsive "cold tumor" colorectal cancer, demonstrating its potential as a next-generation IO cornerstone drug. The company is continuing to follow up on the Phase Ib/II expansion clinical studies of these cancer types. Based on PoC data and regulatory communication feedback, it is expected to take the lead in launching key registration clinical studies in China in 2025 for IO-naïve advanced melanoma and IO-resistant advanced squamous cell lung carcinoma. At the same time, Phase II clinical studies in the United States are underway, and will further expand the research cohorts in lung cancer, colorectal cancer and melanoma.
At the same time, the company is accelerating global innovation through external cooperation. At the beginning of 2025, the company reached a global exclusive licensing agreement with Roche for a total amount of more than US$1 billion. Combined with Roche's strong strength in global R&D, production and commercialization, it accelerated the development of IBI3009 (potential best-in-class DLL3 ADC) to benefit patients with small cell lung cancer around the world.
Looking forward to 2025, high-potential drugs seize the opportunity
Looking forward to 2025, the company expects that it will be a year of gathering momentum and potential explosion. The performance is expected to continue to grow rapidly, innovation strives for breakthroughs, ushering in the launch of 6 new drug varieties, and the commercialization of oncology and comprehensive product pipelines goes hand in hand. At the same time, further improve marketing efficiency and output, especially:
Masudupeptide (GCG/GLP-1): Weight loss and type 2 diabetes indications are expected to be approved for marketing in the first half and second half of 2025, respectively. It will provide a breakthrough innovative dual-target GLP-1 drug with "natural dual targets, fat burning and liver protection, high-quality weight loss, and comprehensive benefits" for the obese, overweight and diabetic population; Tetuinumab
It is expected to be approved for marketing in the first half of 2025, becoming the first anti-IGF-1R monoclonal antibody to be marketed in China. The breakthrough therapy is expected to fill the gap of 60 years of new drugs available in the treatment of thyroid eye disease (TED) in China, directly attacking the root cause and significantly improving exophthalmos and diplopia; Piconcibazumab
It is expected to be approved for marketing around the end of 2025. It is the only IL-23p19 monoclonal antibody in the world with a proportion of more than 80% of subjects achieving PASI 90 (improvement of Psoriasis Area and Severity Index ≥90%) after 16 weeks of treatment. It has clear advantages such as long-term efficacy maintenance, continued effectiveness after IL-17 resistance, and quarterly dosing intervals, which will bring the best comprehensive benefits to Chinese psoriasis patients.
At the same time, the company focuses on its cornerstone products in the fields of CVM, autoimmune disease and ophthalmology - Masudutin, Piconazole and Tetutumab, implements a life cycle management strategy, focuses on patient needs, and plans to extend the development of multiple indications to maximize the value of the product portfolio.
In 2025, the company plans to submit new drug applications for 7 drugs or is in key clinical trials, and 7 innovative pipelines plan to start key registration clinical trials (based on PoC data), which will continue to provide strong momentum for the company's rapid growth. In addition, innovative molecules with global potential will read out PoC data one after another, and new targets and new technology products will enter clinical trials, moving towards the vision of "becoming a world-class biopharmaceutical company" in 2030.
https://news.yaozh.com/archive/44835.html
By editorRead more on
- Phase III clinical trial of vetcotozumab completes patient enrollment February 9, 2026
- The first long-acting coagulation factor VIII, has officially entered the Chinese mainland market. February 9, 2026
- 17.9 billion yuan! A top-selling topical medication emerges. February 9, 2026
- Turnaround! Generic drug giant successfully “revived” February 9, 2026
- Novartis’s first-ever autoimmune drug has been submitted for marketing approval in China. February 9, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



