Latest! “4 innovative medical devices” approved for marketing
December 25, 2024
Source: drugdu
 231
231
 Recently, the National Medical Products Administration approved the registration applications for four innovative products: "Intestinal Polyp Electronic Lower Gastrointestinal Endoscopy Image-Assisted Detection Software" by Changsha Huiwei Intelligent Medical Technology Co., Ltd. (hereinafter referred to as Changsha Huiwei Intelligent), "Rotational Ablative Treatment Device" and "Disposable Coronary Ablative Catheter" by Shanghai Microport Melody Medical Technology Co., Ltd. (hereinafter referred to as Microport Melody), and "Disposable Magnetic Electric Positioning Pressure Monitoring Pulsed Electric Field Ablation Catheter" by Hunan Apt Medical Devices Co., Ltd. (hereinafter referred to as Apt Medical). Another product ofChangsha Huiwei Intelligent has been approved
Recently, the National Medical Products Administration approved the registration applications for four innovative products: "Intestinal Polyp Electronic Lower Gastrointestinal Endoscopy Image-Assisted Detection Software" by Changsha Huiwei Intelligent Medical Technology Co., Ltd. (hereinafter referred to as Changsha Huiwei Intelligent), "Rotational Ablative Treatment Device" and "Disposable Coronary Ablative Catheter" by Shanghai Microport Melody Medical Technology Co., Ltd. (hereinafter referred to as Microport Melody), and "Disposable Magnetic Electric Positioning Pressure Monitoring Pulsed Electric Field Ablation Catheter" by Hunan Apt Medical Devices Co., Ltd. (hereinafter referred to as Apt Medical). Another product ofChangsha Huiwei Intelligent has been approved
Changsha Huiwei Intelligence was established in 2020 and is a wholly-owned subsidiary of Suzhou Huiwei Intelligent Medical Technology Co., Ltd. (hereinafter referred to as Huiwei Intelligence). Huiwei Intelligence was established in June 2019 and specializes in the research and development, production and sales of intelligent medical products. Driven by its independent core technologies in the fields of "artificial intelligence" and "edge computing", the company is committed to providing "high-level, good experience" medical products and services to global medical institutions, helping doctors to improve their diagnosis and treatment levels and efficiency to the greatest extent.
In mid-2021, Huiwei Intelligence's AI-assisted detection system for gastrointestinal lesions-endoscopic "Frame Detection" was officially launched. This product can achieve full functional coverage of upper and lower gastrointestinal lesions detection, assist in detection and lesion classification through high-quality algorithms and computing power, and automatically identify, label, and prompt lesions during endoscopic examinations and surgeries, with extremely high sensitivity and specificity.
In addition, another product of Huiwei Intelligence, the fundus screening product-"Fundus Detection" is also widely praised. Its portable DR fundus screening AI product overcomes many difficulties in AI screening equipment entering the grassroots level. The equipment is lightweight, mobile, has strong software compatibility, superior performance, comprehensive functions, fast computing speed, and controllable dependence on cloud platforms and networks.
In addition to endoscopic-assisted diagnosis and treatment solutions and ophthalmic-assisted diagnosis and treatment systems, relying on the team's strong R&D capabilities, Huiwei Intelligence has mature solutions for other diseases, including lung disease auxiliary diagnosis and treatment products, rehabilitation and elderly care AI products, etc.
The intestinal polyp electronic lower gastrointestinal endoscope image-assisted detection software approved for listing this time is installed and used in a single machine in the form of a U disk. It consists of a user module, a management module, an image acquisition and display module, a regional acquisition control module, an automatic identification module, an algorithm module and a basic module. It is mainly used for adult colon endoscopy.
In addition, the product uses a dual-stream fusion deep convolutional neural network algorithm, which can realize real-time display of dynamic information during surgery. It has the advantages of low latency and high precision, and can provide patients with more efficient and high-quality auxiliary detection services.
Minimally invasive melody
fills the domestic gap
MicroPort Melody is a subsidiary of Shanghai MicroPort Medical Device (Group) Co., Ltd., a subsidiary of MicroPort Medical Science Co., Ltd. It is an enterprise mainly engaged in technology promotion and application services. It has independently developed from the field of minimally invasive coronary artery and is committed to the research and development of active Class III coronary medical devices. Many products currently under development are domestic innovative coronary medical devices, breaking the market monopoly of foreign products, and achieving better performance and better treatment effects with the working principle of high-tech products.
In coronary intervention, severe coronary calcification lesions have an important impact on the success rate of surgery and patient prognosis. In the face of hard calcified lesions, atherectomy pretreatment can help open the lumen and modify the calcified lesions, facilitating the passage of subsequent instruments and stent expansion and wall adhesion.
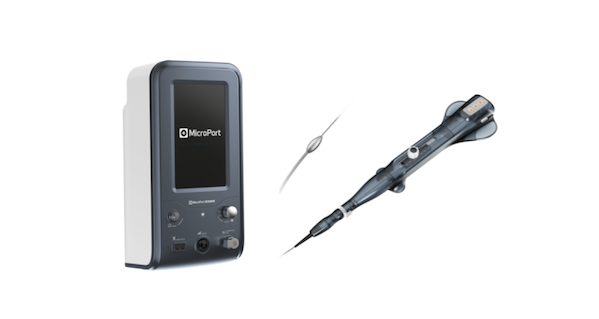
MicroPort Melody™'s coronary atherectomy system fills the gap in the field of atherectomy for domestic instruments. It has a atherectomy head design with an eccentric structure that is the first in China. During use, the centrifugal force can be changed by adjusting the rotation speed, thereby controlling the atherectomy diameter, reducing the replacement of instruments during surgery, and bringing better atherectomy effects. At the same time, the entire surface of the grinding head is fully covered with diamonds, which can be used for bidirectional atherectomy in the axial direction to reduce the risk of grinding head incarceration.
In terms of appearance, the propeller of this product adopts a unique butterfly tail design to increase the contact area and make it more stable. At the same time, the translucent material makes the product look softer, and the color of the whole system uses the Blue Crane series, which represents the feeling of "healing". In terms of control, the product adopts a highly integrated and portable design concept, making it easier to use in clinical applications.
At the beginning of this year, this coronary atherectomy system has won two major awards in the design industry: the "Red Dot Product Design Award" and the "iF Design Award". In September, it won the "Shanghai Design 100+" award, once again demonstrating the strong strength of MicroPort®'s innovative research and development and industrial design.
After approval for listing, the FireRaptor® Coronary Artery Rotational Atherectomy System will work with the spinous process balloon and the coronary pulse shock wave catheter system to jointly build a solution for high-resistance coronary lesions and provide a powerful pretreatment tool for the majority of coronary interventional physicians.
Apt Medical
focuses on minimally invasive cardiovascular intervention
Founded in 2006, Apt Medical is a wholly-owned subsidiary of Huitai Medical. It is a high-tech enterprise focusing on the research, development, production and sales of cardiac electrophysiology and interventional medical devices. It has formed a business layout dominated by complete coronary access and cardiac electrophysiology medical devices, and peripheral vascular and neurointerventional medical devices as the key development direction.
Since its establishment, Apt Medical has always been committed to independent innovation and development in the field of minimally invasive intervention of cardiovascular and cerebrovascular diseases, and will make outstanding contributions to promoting the development of global medical undertakings. More than 20 products have been developed for the diagnosis and treatment of cardiovascular diseases, including catheter sheaths with hemostatic valves, atrial septal puncture systems, super-slip angiography catheters, distal protectors, AAA/TAA stents, PTCA balloon catheters, microcatheters, guide catheters, guide extension catheters, blood flow reconstruction devices, and thrombectomies for the treatment of cerebrovascular diseases. The
disposable magnetic-electric positioning pressure monitoring pulsed electric field ablation catheter approved for marketing this time consists of a head end, a tube body, a handle, a connecting cable, and an optoelectronic hybrid plug, and is used in conjunction with the cardiac pulsed electric field ablation device of Shanghai Hongtong Industrial Co., Ltd.
Shanghai Hongtong's cardiac pulse electric field ablation device was also recently approved for marketing (! "2 innovative medical devices" approved for marketing). The product consists of a main unit, an isolated power supply, a foot switch and accessory cables. The accessory cables include: three-dimensional positioning device connection cable, serial computer interface cable, impedance monitoring connection cable, and grounding cable. This product is used in conjunction with a disposable pulse electric field ablation catheter and is mainly used for the treatment of atrial fibrillation.
In addition, the product uses pulse electric field ablation technology to selectively treat the tissue at the lesion site by controlling the energy of the pulse electric field, causing irreversible electroporation damage to myocardial cells, thereby achieving the treatment goal. This technology can effectively reduce the risk of thermal damage to surrounding normal tissues, so that more patients with drug-refractory, recurrent, symptomatic, and paroxysmal atrial fibrillation can benefit.
In December, 8 innovative medical products have been approved for marketing. More and more domestic innovative forces are emerging. Innovation, as the first driving force, is continuously leading the continuous rise of my country's medical device level. As the pace of innovation in China's medical devices accelerates, more innovative products will be launched in the future, and China's medical device industry is accelerating towards the goal of high-quality development.
https://news.yaozh.com/archive/44714.html
By editorRead more on
- Phase III clinical trial of vetcotozumab completes patient enrollment February 9, 2026
- The first long-acting coagulation factor VIII, has officially entered the Chinese mainland market. February 9, 2026
- 17.9 billion yuan! A top-selling topical medication emerges. February 9, 2026
- Turnaround! Generic drug giant successfully “revived” February 9, 2026
- Novartis’s first-ever autoimmune drug has been submitted for marketing approval in China. February 9, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



